Meet us at BIO
Phase 0 taking the industry by storm
Save your seat at the next BIO
Phase 0 data points
• Approved method by FDA and EMA
• Up to a 73% higher chance of success in subsequent trials
• First patient in within 6 months,
• On- /off-target data in approximately 12 months
• GLP material is sufficient
• Only limited toxicity studies needed
• Skip large animal studies
• Significantly lower investment in comparison to Phase I
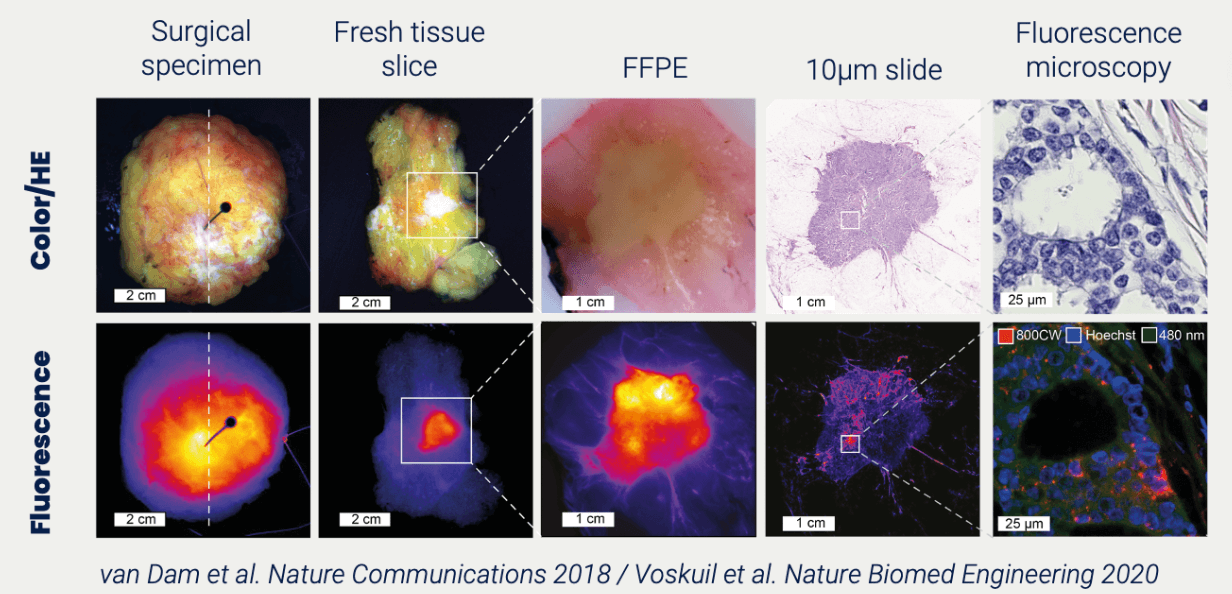
Phase 0 taking the industry by storm
90% of new drugs never reach the market. Most of them fail in the more expensive phases of clinical trials. Leaving drug developers with empty hands after sometimes more than a decade of research. Phase 0, combined with molecular imaging, provides you as a drug developer with reliable PK/BD data. At TRACER we test your novel drug directly in your target population.
The huge benefits have resulted in Phase 0 taking the industry by storm. Many drug developers are already using it for their pipeline. TRACER’s team has more than 30 years of experience in molecular imaging and clinical trials. Our method can be applied to almost all types of compounds.
Let’s meet
Are you attending the next BIO? Book a meeting to determine the effect of Phase 0 on your pipeline. If not, contact us to book an online meeting outside the conference.
Schedule a meeting
© 2024 TRACER | Aarhusweg 2-1, 9723 JJ Groningen, The Netherlands | info@tracercro.com