An in-human microdosing study allows drug developers to research their new drug in patients before Phase 1. Microdosing in Europe, the US, and other regions have been supported for more than 20 years by, respectively, the EMA and FDA and are harmonized under ICH guidance. In recent years, microdose studies have gained traction under the name of Phase 0 microdosing, exploratory trials, and early Phase 1 microdosing trials. TRACER specializes in early clinical research studies and, as a CRO, conducts microdosing clinical trials. In this blog, we introduce you to all aspects of microdosing in early clinical research.
What is microdosing in clinical trials?
Let’s start by answering some basic questions about microdosing in clinical trials. What is microdosing in clinical trials? Simply put, a microdose is a very low, sub-therapeutic dose of a new drug. By using this low dose in clinical trials, regulations allow for in-patient testing at the earliest stage of drug development. This is all with a limited animal data package, without Phase 1 safety studies, and in some cases even using GLP-grade drug substances. In other words, a microdosing study is offering drug developers an affordable and valuable tool to obtain critical data for informed decision-making before Phase 1/2.
When are in-human microdosing studies conducted and in which clinical phase?
For most of our sponsors, in-human microdosing studies are part of their early phase research, but for others, we conduct it in parallel/after their Phase 1b or 2a study. As per ICH M3 R2 guidelines, a microdose study can be initiated with a minimum of animal data. Often a 14-day single-dose extended toxicity study is sufficient. Microdosing in clinical trials is a unique solution, you can immediately conduct research in patients and skip extensive animal research and don’t have to wait on Phase 1 healthy volunteer studies.
– Famke Van Renesse-Brouwer, MSc, Business to Science Manager
How much is a microdose?
Guideline ICH M3 R2, defines a microdose as:
- Total dose ≤ 100 μg (no inter-dose interval limitations)
- AND total dose ≤ 1/100th No Observed Adverse Effect Level (NOAEL)
- AND ≤1/100th Pharmacologically Active Dose (PAD) (scaled on mg/kg for i.v. and mg/m2 for oral)
- Whichever of the above is lowest
- For protein products, ≤ 30 nmol (introduced by the FDA in 2006, and referenced by EMA for large molecules)
The use of imaging in Phase 0 microdosing
From a microdose, no therapeutic effect can be expected. Therefore, sensitive detection methods, like nuclear imaging, are often necessary to provide valuable clinical data. Nuclear imaging combined with blood sampling can provide data on pharmacokinetics (PK), biodistribution (BD), and target engagement. Even more so, with dosimetry calculations microdose data can be extrapolated to predict therapeutic dose behavior in target tissues and organs. This data can be used to attract funding and is valuable as a derisking mechanism for investors.
Other methods to obtain data for microdose studies
In addition to nuclear imaging, optical imaging (e.g. fluorescence, Raster-Scan Optoacoustic Mesoscopy (RSOM)) can be used. But also without imaging, the presence and concentration of the drug substance can be measured. Examples are RAMAN spectroscopy, Liquid Chromatography with tandem Mass Spectrometry (LC-MS/MS), and Accelerator Mass Spectrometry (AMS).
Microdosing case study: VAR2 Pharma
Comparing the binding and biodistribution of two scFv antibody fragments in multiple solid tumor types. Both fragments have shown promising results in preclinical testing. In-patient data can be decisive in choosing a lead compound for further research. The Phase 0 microdosing study conducted by TRACER includes 16 patients per candidate with various solid tumor types. From this study design, biodistribution and tumor accumulation can be compared before these antibody fragments are converted and used as Antibody Drug Conjugates (ADCs).
Microdosing studies are a new phase of clinical research
Microdosing trials fall under a new category of research: exploratory in-human research. In Clinicaltrials.gov these studies are defined as early Phase 1. Although, it should be said that microdose studies can also be found in other phases of drug development. For exploratory trials, the ICH defined five types. Of these five, two use a microdose and three use higher doses. You can find and compare all types of exploratory research in our blog on ICH M3 R2 guidelines for exploratory studies.
The lowest preclinical requirements to move into the clinic
Regulatory agencies have high expectations for exploratory studies and have a more flexible approach when it comes to preclinical and Chemistry Manufacturing and Control (CMC) requirements. A microdose study can already be conducted with only a 14-day extended single-dose toxicity study. So without other toxicity studies and without safety studies in healthy volunteers. This is possible because the administered dose is well below the pharmacologically active range and is therefore considered non-therapeutic and low risk.
CMC requirements for microdosing studies
The regulations are also less stringent in terms of CMC requirements if the drug falls under a certain predefined risk. For imaging studies, if the labeled drug product is produced in a Good Manufacturing Practice (GMP) facility, the precursor – the drug substance – can be of Good Laboratory Practice (GLP)-grade. This decreases timelines and lowers the cost of producing the clinical trial material and lowers the microdosing clinical trial cost in general. For a Phase 1 study, the Investigational Medicinal Product (IMP) still needs to be fully GMP (for both precursor and drug product). However, this pathway allows you to obtain in-human data and make changes to the compound based on that data before producing the costly GMP IMP. Once the first data is obtained, you can, however, already start producing GMP material to save time.
The urgent need for microdosing in clinical trials
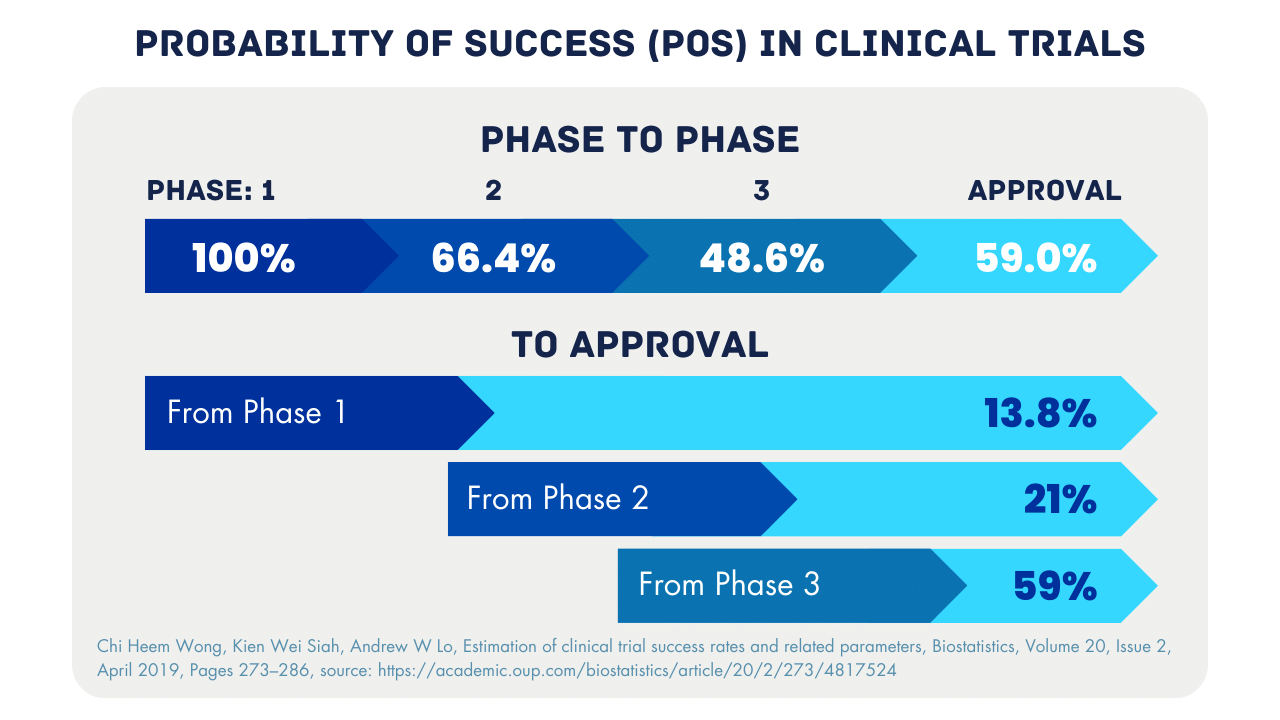
Many drug developers are still used to the conventional pathway in the early stages of clinical trials, that is, after R&D a preclinical pathway, Phase I in healthy volunteers (in certain cases in-patient), followed by Phase II in patients. However, everyone is aware of the high failure rates in clinical drug development. Multiple studies have shown a low Probability Of Success (POS) between the phases. The aim of Phase 0 microdosing studies is to improve the selection of candidates entering further phases of clinical trials.
Why do clinical trials fail?
Many compounds fail because they cannot demonstrate efficacy in patient studies. Although the R&D and preclinical data were promising, why did they fail in Phase II? At TRACER, we believe the lack of in-patient data – to be used for lead compound selection and study design – is an important factor. We develop drugs for patients, so why design in-human studies based on animal data? That is exactly why we at TRACER developed the Phase 0 microdosing solution based on the regulatory framework. We deliver the missing data that drug developers can use for drug candidate selection, patient stratification, and clinical trial design. All factors that contribute to future success.
Learn more about microdosing studies
Are you curious about the potential of microdosing studies for your research? Or are you an investor interested in learning more about exploratory trials as a derisking mechanism? As specialists in these types of studies, we at TRACER are happy to answer these and other questions. Contact us, send us a message or request a meeting.
Request more information
or send an e-mail to info@tracercro.com
FAQ on microdosing
We have explained the concept of microdosing. Below we answer some common questions we often get when we explain microdosing studies to people at conferences.
What is a cassette microdose study?
The result of a microdosing study is PK and BD data of your drugs from patients with the targeted disease and even different disease subtypes. When testing multiple drug candidates, the study type is called cassette microdosing. You can test up to five compounds, with a total volume not higher than the microdose definition, to make sure you choose the most promising lead for further clinical research. A key difference from other solutions is that all decisions are now based on in-patient data rather than animal data or models.
How quickly can multiple doses be administered?
For multiple dosing in a microdosing study, time limits apply. In general, a new dose may be administered only after the previous dose has been cleared from the system. When a compound is labeled, the half-life of the radionuclide also plays a role. For example, for Carbon-11, with a half-life of around 20 minutes, administering multiple doses is easier than a radionuclide with a long half-life.
How much protein can be expressed?
For protein products, the microdose limit is set at 30 nmol. For antibodies (based on 150 kDa), the total amount of protein (in mg) that can be administered under microdosing regulations correlates to a flat-dose of 4,5 mg. But what about antibody fragments? This depends on the size. For a protein of 100 kDa a flat-dose of 3 mg would apply. The calculation below shows why.
Mass = (3.0 × 10⁻⁸ moles) × (100,000 g/mol) = 3.0 × 10⁻³ g = 3.0 mg
Step-by-step calculation
Convert nanomoles to moles:
30 nmol = 30 x 10⁻⁹ mol
Use molecular weight to convert moles to grams:
The molecular weight (MW) of the protein is 100 kDa.
Since 1 kDa = 1,000 g/mol, then:
100 kDa = 100,000 g/mol
Now calculate the mass:
mass in grams = 30 x 10⁻⁹ x 100,000 = 3.0 x 10⁻³ g
Convert grams to milligrams:
3.0 x 10⁻³ g = 3.0 mg flat-dose
If you have questions, or if you need help calculating your protein size to flat-dose, contact TRACER.
Does the microdose apply to the drug product or only to the drug substance?
The microdosing limit applies to the precursor/drug substance. Not the labeled product (radionuclide) or drug product. The drug product may include excipients, chelators/linkers, fillers, buffers, etc.
Can a microdose be too small to detect anything?
You visualize the imaging agent, coupled to the compound – not the compound itself. For radionuclides there are predefined doses that can be used, and those are sufficient for imaging. Cameras for optical and nuclear imaging are very sensitive. So, a microdose is sufficient for clear-resolution imaging.
Can the data be used for the FDA and EMA?
Yes, data of a microdosing trial can be used. With your exploratory trial submission, you need to define clear objectives and analytical methods. If you’re planning to use microdose data in a regulatory submission, it’s best to discuss this with TRACER. Especially if you want a more flexible trial with an adaptive design. Regulations allow in early phases these more flexible approaches. Data from microdosing studies cannot serve as proof of efficacy but do contribute to the proof of concept. For further study designs, microdosing data can be used. For the FDA, TRACER can support in Clinical Data Interchange Standards Consortium (CDISC) programming of your data.
What are the broader benefits of a microdosing study?
The concept of microdosing study was introduced to lower the failure rate of drugs in clinical trials. But the benefits are broader than that. The result is that fewer resources – lab animals, trial participants, time, and money – are spent on drugs that would otherwise ultimately fail. With Phase 0, those resources remain available for promising compounds. Furthermore, these high promising compounds can be included in better-informed and thus better-designed clinical trials. The objective of Phase 0 microdosing studies, and the mission of TRACER, is to move drugs faster from bench to bed, simply by improving research with better data.