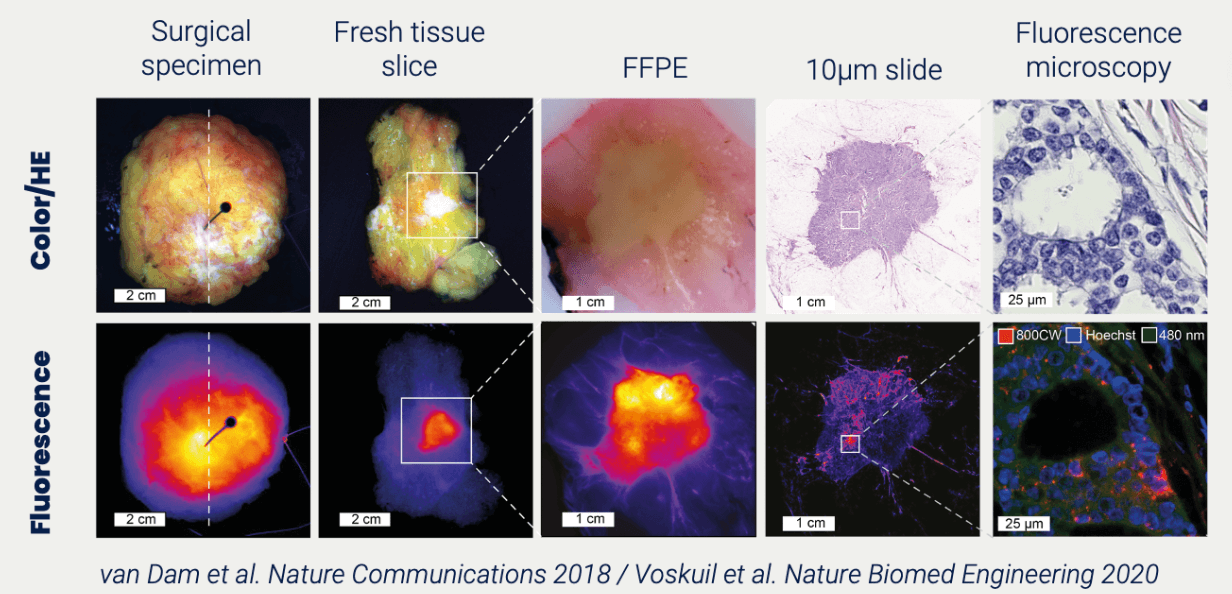
Proof of Concept Study: get in-human PK/BD data
A Proof of Concept study, PoC study for short, has two main objectives. One is that with a Proof of Concept clinical trial in humans, you find out if your new compound has positive PK/BD data. This means high uptake in target tissue and risk analysis for off-target side effects. The other is that a positive outcome from your PoC study, Proof of Concept trial, can make your compound more attractive for further investment. Proof of Concept studies are first in human clinical trials before the Phase 1 trial. Therefore, they are often referred to as Phase 0.

Proof of concept clinical trial
At TRACER we specialize in Proof of Concept research using molecular imaging, with a strong focus on first-in-human PoC. In this article, you will read about the design of a Proof of Concept study. To get a quick understanding, we go over the main details of the Proof of Concept clinical trial with microdosing.
Get to know more about PoC study for drug development
Phase 0 data points
• Approved method by FDA and EMA
• Up to a 73% higher chance of success in subsequent trials
• First patient in within 6 months,
• On- /off-target data in approximately 12 months
• GLP material is sufficient
• Only limited toxicity studies needed
• Skip large animal studies
• Significantly lower investment in comparison to Phase I
POC study
The FDA approved the Phase 0 POC study in 2006 to solve the fact that 90% of new drugs never reach the market. The goal of a Proof of Concept trial is to prevent new compounds from failing in the more expensive phases of clinical trials.
Note: The Proof of Concept study meaning depends on the method used. Instead of only measuring drug concentrations, a Phase 0 study combined with molecular imaging provides visual, exact, and reliable PK/BD data. At TRACER we test your novel drug using this method directly in your target population.
Proof of Concept trial
Are you working at a biotech or a pharmaceutical company that is considering a PoC study? It’s good to know that many drug developers are already using it for their pipeline. TRACER’s team has more than 20 years of experience in molecular imaging and clinical trials. Our method can be applied to almost all types of compounds.
Request information about a PoC study for your compound.
Request more information
Proof of Concept study design
A proof-of-concept clinical trial design generally includes the following steps:
1. Proof of concept research question
First, you need to define your research question or hypothesis. Keep in mind that a PoC study is always conducted with a subtherapeutic dose on patients. Your research will therefore focus on indication (or potentially a specific mutation), biodistribution, on- and off-target uptake, and the areas at risk for potential side effects. Therapeutic effects cannot be achieved in a Proof of Concept trial, but the retrieved data will be beneficial in further clinical trials.
2. Conjugating your compound with a label
To retrieve PK/BD data you need to find a label that you can conjugate to your compound without affecting its characteristics and allowing good measurement in vivo. The labeled compound (tracer) will show absorption, distribution, metabolism, and excretion.
3. Proof of Concept clinical trial protocol development
In this step, you will set the outlines for your study. Meaning:
• Set up inclusion and exclusion criteria;
• Choose your type of clinical trial;
• Choose the location(s) for your trial;
• Define your sample size;
• Define trial duration;
• Choose methods of data collection and validation;
• Define outcome analysis.
To learn more, download a clinical trial protocol template or example.
Trial feasibility
An important part of this step that is often overlooked is feasibility. It may happen that a clinical trial fails due to strict in- and exclusion criteria for the patients or a protocol design that is not feasible or a too large burden for the patient (and as a result patients refuse to participate). Don’t hesitate to use our 30 years of experience in this field. Contact us to discuss the best Proof of Concept examples related to your novel drug.
4. Regulatory approval on your Proof of Concept clinical trial
You will need regulatory approval before you can conduct your PoC clinical trial. Choosing to conduct your Clinical trials in The Netherlands has many benefits, including fast regulatory approval. The regulations for PoC clinical trials are different than for the other phases of clinical trials.
– You don’t need extensive tox data, in most cases an extended single dose toxicity study is sufficient. In case toxicity data of your compound is available and the label we use for molecular imaging is known, this step might not even be obligatory.
– You only need GLP material, as at TRACER we will label the GLP material in a GMP (hotlab) facility to manufacture a GMP product for use in PoC trials.
5. Conducting your Proof of Concept clinical study
At step 4 you’ve designed the Proof of Concept study, obtained regulatory approval and are ready to move into the execution of the first in human clinical trials. Meaning the recruitment of participants, data collection, analysis, safety monitoring and interpretation and reporting.
Please note that depending on your PoC study design and the country you are conducting your trial, your PoC clinical trial may be different. Therefore, we would like to get in contact so we can advise you regarding the design for your study.
Contact TRACER now or read below the FAQ on this subject.
Frequently asked questions about Proof of Concept study
There are several questions about the PoC study we often receive. Take your time to read the relevant questions for you or contact us regarding other information.
Is the Proof of Concept study FDA approved?
The Proof of Concept study referring to the Phase 0 POC clinical trial is approved by the FDA. The focus of Phase 0 first in human clinical trials is to evaluate the targeting of a new drug in patients. Depending on the Proof of Concept study level of evidence, there can be a go/no-go decision made to move a drug into subsequent clinical trials.
What phase is a Proof of Concept study?
A Proof of Concept trial is also known as Phase 0. Meaning the PoC clinical trial comes before Phase I-IV. It is a clinical study and the first in human clinical trial.
What is the biggest difference between Proof of Concept clinical trial – Phase 1
Comparing Proof of Concept clinical trial with Phase 1, the cohort is one of the major differences. Where Phase 1 is conducted on a large number of healthy volunteers, the PoC study is conducted on a small number of patients (+/- 10). Since PK/BD data can differ between healthy volunteers and the target population, data from the Proof of Concept clinical trial can be more reliable than Phase 1.
Can Proof of Concept benefit clinical trial Phase 2?
Phase 2 in clinical trials has the objective to test your drug’s efficacy. Because you already investigate this with your PoC study, you can move with more confidence into this phase of clinical trials. You also have a better understanding of the indication your drug targets. This means you can select your participants better, get more reliable data, and your chances of success increase.
What is PoC vs PoV?
As you may know by now, PoC stands for Proof of Concept, but what is the meaning of PoV and how are they different? PoV stands for Proof of Value, meaning the business value of your concept. Therefore, PoV will focus on financial and strategic benefits. The difference between a PoC and a PoV is that the PoC has a narrow, technical-orientated scope, while PoV has a broader scope focused on business value.
What is an example of a Proof of Concept?
If you are looking for Proof of Concept examples, then you should read our case study in oncology.
How do you write a Proof of Concept study?
How do you write a Proof of Concept study? In general, you outline the purpose, methodology, and expected outcome. A structure that can be used is the following:
• Introduction
• Methodology/study design
• Execution/Implementation
• Data analysis
• Results and discussion
• Conclusion
Remember what your main goal for the Proof of Concept study is: gathering data that prove the mechanism of your novel drugs. The Proof of Concept study design and sample size both depend on what proof you need.
Consult TRACER
Even if you are currently not ready for the phases of clinical trials yet, you can already consult with us. Contacting TRACER early in your drug discovery process might allow you to skip steps in preclinical development. A Proof of Concept will identify the most promising compounds in your drug discovery pipeline at an early stage in your R&D.
Contact TRACER