Radiation dosimetry in early-phase clinical trials encompasses safety, dosing, distribution, absorption, and pharmacokinetics. It applies to radiotherapy, radiopharmaceuticals, and labeled compounds. A radiation dosimetry study is required, whether external or internal radiation is used and whether it is a diagnostic agent or therapy. Factors for measurements and calculations are radionuclide, ligand, carrier molecule, and intended target. A TRACER dosimetry clinical trial, or as part of your study, provides internal dosimetry data to determine the administration dose, absorption in diseased and healthy tissues and organs, exposure over time, and potential side effects. Read more about preclinical and clinical dosimetry or contact TRACER.
Contact TRACER
Key points of dosimetry studies
Dosimetry studies evaluate the radioactive dose delivered to and absorbed in diseased and healthy tissue and organs. The key points of these dosimetry trials are listed below.
- Dosimetry studies assess radiation safety.
- For diagnostics, the quality of its measurements is evaluated.
- For therapeutics, optimal dosing is determined for efficacy. E.g. tumor response.
- Calculations are done to estimate dosing, including an uncertainty analysis.
- Quantitative imaging delivers data on the biodistribution and in-vivo activity over time.
- Clinical trials for radiopharmaceuticals can deliver valuable data on in-vivo stability and the occurrence of (radio)metabolites.
Good to know: dosimetry definition and meaning
There is often a lack of clarity about the dosimetry definition. Dosimetry meaning the assessment of the radiation amount absorbed by material, such as tissue or organs. In drug development, the radiation can be emitted by devices (X-rays or CT for example) or compounds emitting radiation after labeling with a radionuclide. Nuclear medicine dosimetry is required for all types of radioactive compounds and devices.
Dosimetry for radiopharmaceuticals vs devices
For radiopharmaceuticals, the radioactive substance gives radiation exposure to the patient. The amount of radiation exposure depends on how much and how long the radioactivity remains in an organ and the body. This is determined by the biodistribution/clearance and physical decay of the radionuclide. To calculate dosimetry, you need images and blood samples at different times to calculate the amount of radioactivity over time. You can then use this to determine the dose to organs and the body.
This is different from dosimetry with external irradiation. Here you don’t need imaging, because you give radiation from outside for a certain time, with a certain energy on a selected body part (for diagnosis or therapy).
TRACER: (nuclear) labeling of your compound for imaging in clinical trials
TRACER specializes in molecular imaging in clinical trials. Often it is thought dosimetry can only be used for the development of radiopharmaceuticals. However, we at TRACER label new drugs with a radionuclide to investigate the biodistribution of the new drug. Dosimetry can be used to calculate the estimated amount of drug in the target. This is ideal for early phase clinical trials, especially Phase 0. Because labeling the study drug temporarily makes it a radiopharmaceutical, other regulatory rules may apply. We are happy to assist you with the understanding of regulatory requirements to start your clinical trial.
Two types of dosimetry trials at TRACER
In addition to preclinical dosimetry, TRACER provides drug developers with two types of dosimetry clinical trials: Phase 0 and Phase 1. Phase 0 is a fast clinical trial often with a low dose, without therapeutic intent. The Pharmacokinetic (PK) and Biodistribution (BD) data from Phase 0, is useful to design the Phase 1 study. A dosimetry study in Phase 0 can replace the Phase 1 dosimetry trial. The low dose for imaging can be extrapolated to the dose for therapy.
Contact TRACER to make an appointment about what is the best first human trial for you.
Request meeting: Dosimetry for Drug Development
(30 minutes online meeting, no charges apply)
Phase 0 radiopharmaceuticals
One of the benefits of Phase 0, is the ability to obtain early in-patient data with minimal preclinical requirements. By limiting the dose (compound and radiation dose) in Phase 0, participant safety is considered. The limited dose might be the same as the considered dose for diagnostic radiopharmaceuticals. For therapeutics or theranostics, this smaller dose used for imaging is sufficient for an early assessment of safety and efficacy. The radiation dose absorbed by tissue and organs can be made visible with imaging and estimated with dosimetry calculations. A higher dose of both compound and radiation or one or the other is possible for Phase 1.
Radiopharmaceutical clinical trials: FDA and EMA preclinical guidelines
Radiopharmaceutical clinical trials are highly complex. Regulatory agencies, such as the FDA and EMA, demand robust data for dosimetric evaluation for clinical trials. FDA CFR 21 states: “…sufficient data from animal or human studies to allow a reasonable calculation of radiation-absorbed dose to the whole body and critical organs upon administration to a human subject. Phase 1 studies of radioactive drugs must include studies which will obtain sufficient data for dosimetry calculations.” TRACER can inform and advise you on the preclinical necessities. Schedule a meeting with us to discuss the (pre)clinical steps to bring your radiopharmaceutical into clinical trials as fast as possible.
Regulations on clinical trials for radiopharmaceuticals
For translational clinical trials, the harmonized regulations ICH M3 guideline provides guidance on non-clinical safety studies. It lists the required toxicology studies for pharmaceuticals, including radiopharmaceuticals. Listed below are several other regulatory documents of importance. Ahead of your conversation with TRACER, you can already check the following resources.
- EANM guidance document: dosimetry for first-in-human studies and early phase clinical trials
- SNMMI MIRD Pamphlet 11, which can be found in all MIRD material
- EMA Radiopharmaceuticals – Scientific guideline
- EMA Clinical evaluation of diagnostic agents – Scientific guideline
- EMA Guideline on the non-clinical requirements for radiopharmaceuticals
- FDA Microdose Radiopharmaceutical Diagnostic Drugs: Nonclinical Study Recommendations
- FDA Nonclinical Evaluation of Late Radiation Toxicity of Therapeutic Radiopharmaceuticals
- FDA Oncology Therapeutic Radiopharmaceuticals: Nonclinical Studies and Labeling Recommendations Guidance for Industry
Different requirements on dosimetry studies for diagnostics and therapeutics
Regulators divide radiopharmaceuticals for diagnostics and therapeutics. Diagnostic radiopharmaceuticals have fewer preclinical requirements than therapeutic radiopharmaceuticals. Drug developers are always required to evaluate the toxicity, estimate absorbed radiation dose, and justify the efficacy-potential of the radionuclide and ligand or carrier component. To do so, accurate measurement of the radiation dose absorbed by tissue and organs is required. A combination of imaging, bioassays, and calculations via software are included in these clinical trials.
Clinical trial documentation for radiopharmaceuticals
The clinical trial submission documentation for radiopharmaceuticals should include data on chemical and pharmaceutical properties, production, radioactive characteristics, safety, efficacy, toxicology, and dosimetry. Preclinical toxicity studies may be omitted for no-carrier radioactive components for which toxicity is known. This data is needed in for example the Investigational Medicinal Product Dossier (IMPD), Clinical Trial Application (CTA), Investigator Brochure (IB), and Investigational New Drug Application(IND). Schedule a meeting if you need to discuss what applies to your clinical trial. We can help you prepare, write, or review documentation to start your clinical trial as soon as possible.
Gamma, beta, and alpha-emitting radioisotopes
In the last decade, a renewed interest in radiopharmaceuticals has emerged in the form of targeted radionuclide therapy. In diagnostics, the low energy gamma ray emitting radioisotopes like Technetium-99m for SPECT or positron emitters for PET (such as F-18, Ga-68 or Zr-89) are used. In therapy, beta and alpha emitters can be used to destroy cancerous cells. Examples for beta- and alpha-emitters are Lutetium-177 and Actinium-225, respectively. In both therapy and diagnostics, the binding agent delivers the radionuclide to the target.
Radionuclides accumulating in diseased tissue
In some cases radionuclides itself accumulate in diseased tissue. Examples are radioisotopes of iodine for the diagnosis and treatment of thyroid malignancies and the alpha-emitter radium-225 for the treatment of bone metastasis. The assessment of selectivity of the radiopharmaceutical should show delivery of an effective dose to the target within safety limits to the rest of the body.
When a radiopharmaceutical is not suitable for imaging, how is dosimetry/imaging done?
New emerging radiopharmaceuticals are not always compatible with existing imaging methods. For example, imaging of alpha emitters is challenging. To overcome this challenge, we can study the binding mechanism of the agent connected to a surrogate imaging radionuclide to provide an early dosimetry calculation.
– Maarten Brom VP Technology Assessment
Dosimetry software list
In recent years more commercially available clinical dosimetry software has become available. Providing an alternative to in-house developed software at academic institutions or hospitals. We list the commonly used software for dosimetry.
- OLINDA/EXM®
- Dosimetry Toolkit® DTK (GE Healthcare)
- PLANET® OncoDose PDOSE (DOSIsoft)
- STRATOS® (Phillips)
- Hybrid Dosimetry Module™ (HDM) from HERMES (OLINDA/EXM® V2.0)
- SurePlan™ MRT (MIM)
- Organ Dosimetry™ (Hermes Medical Solutions)
- Voxel Dosimetry™ (Hermes Medical Solutions)
- QDOSE® (ABX-CRO)
The use of combinations of software is possible. For example, Organ Dosimetry™ and Dosimetry Toolkit® DTK both use OLINDA/EXM® for organ-level absorbed dose (AD). In your IND or IMPD submission, you need to list the used software and version. When you want to compare dosimetry software, many studies can be found. If needed, we can e-mail you relevant studies. Send us a message if guidance is needed.
TRACER dosimetry studies
At TRACER we specialize in molecular imaging clinical trials with radiolabeled compounds. The radioactivity administered to the study subjects causes exposure to organs and the body. To calculate the absorbed dose we perform dosimetry studies, as part of the clinical studies. This provides a better understanding in terms of dose calculation and dose finding for future therapeutic trials.
Factors in dosimetry studies
Finding the optimal dose for a radiopharmaceutical is crucial in terms of efficacy and risk. Factors that are taken into account are:
- Type of radionuclides
- Half-life
- Energy emitted
- Energy absorbed by tissue or organ
- Energy absorbed by the target
- Mass of the target to calculate per unit
- Route of administration
- Number of administrations and administered amount
- Mean Residence Time (MRT)
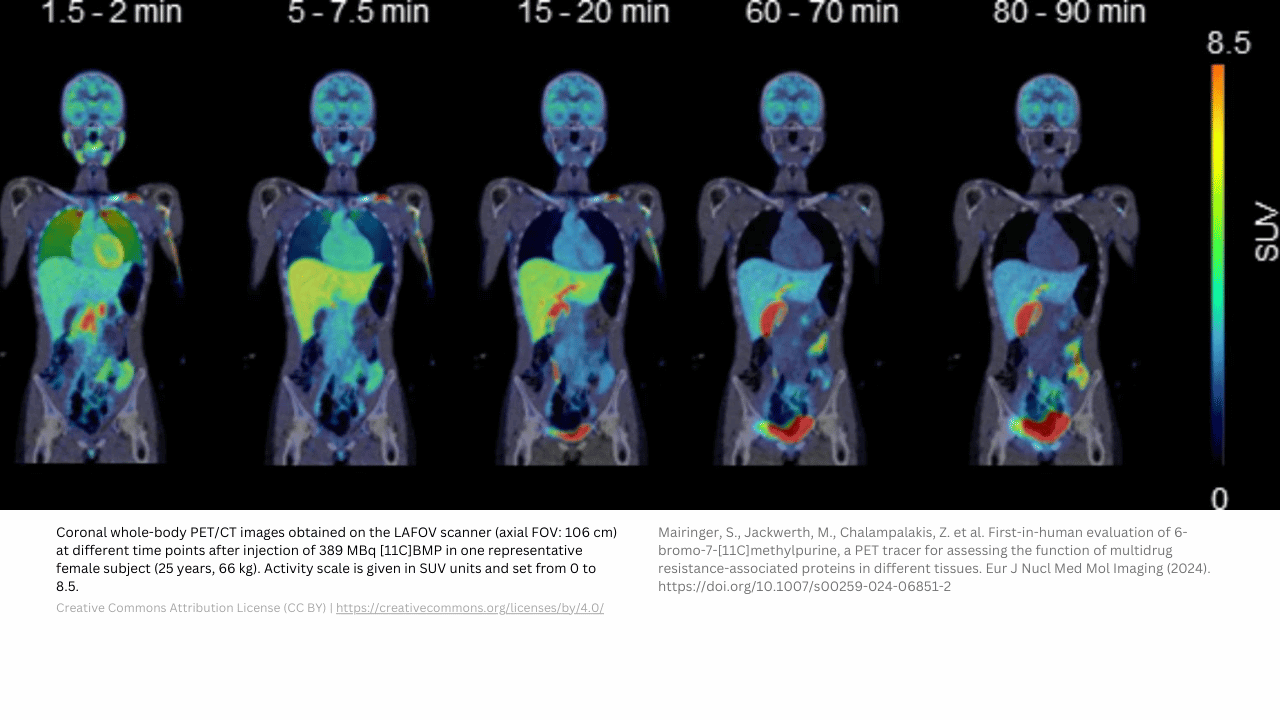
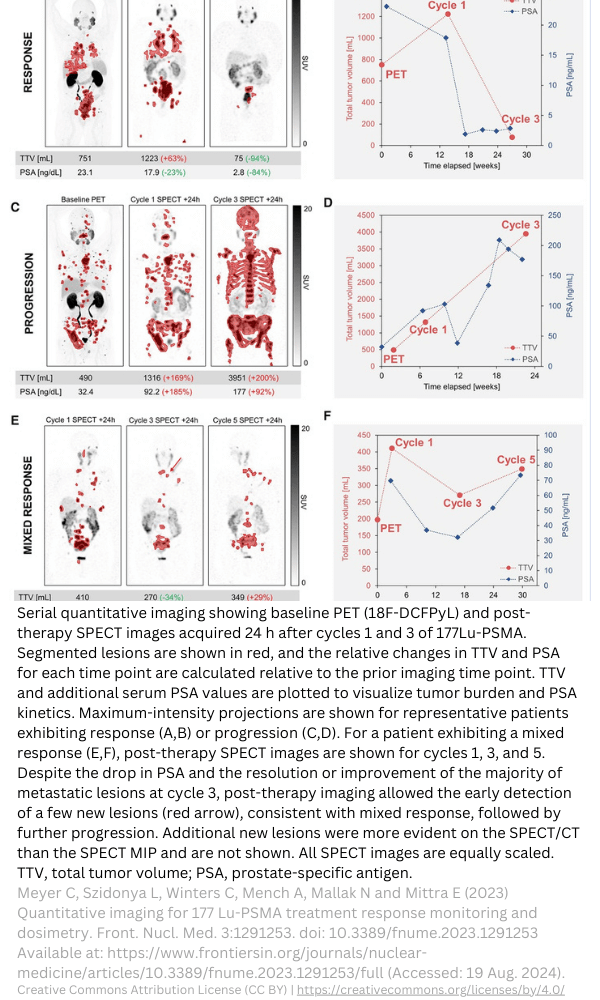
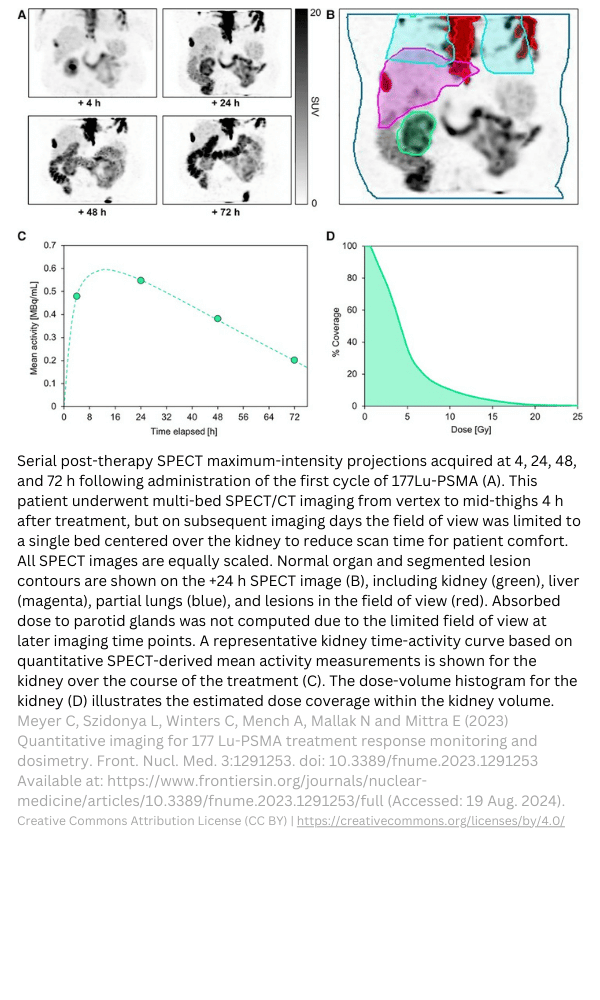
First in human studies: selection of starting dose
Preclinical animal biodistribution and dosimetry data can be used to select the starting dose or Administered Activity (AA). Patient-specific data, such as body weight, surface area, or data from (CT) imaging can be used to deviate from standard anatomic models. TRACER specializes in first in human studies and can assist you with your method selection.
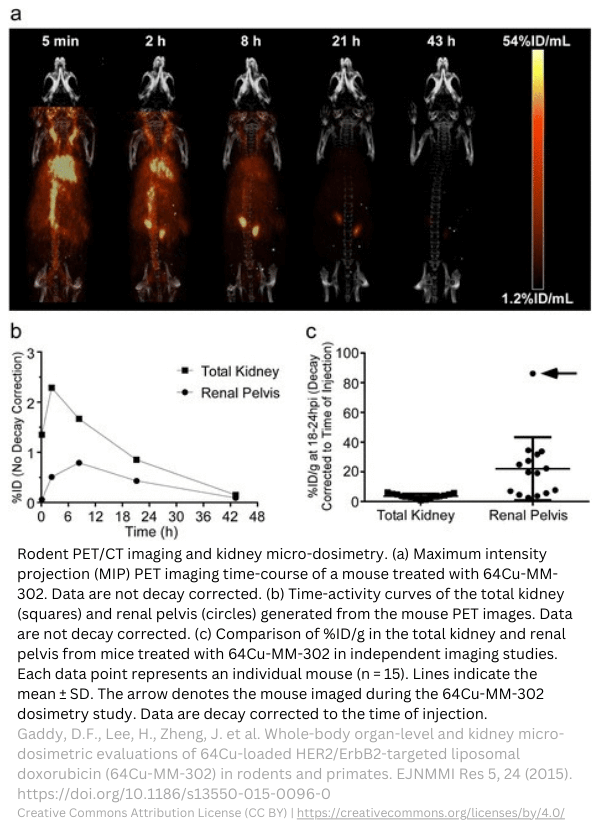
Kidney micro-dosimetry in rodents.
Preclinical dosimetry studies
A preclinical dosimetry study, or thorough estimations of the dose in humans ensures the safety and efficacy of the radiopharmaceutical before allowing testing in humans. The first step to estimating dosimetry is to analyze toxicity. The second step is pharmacologic characterizations for validation of antigens on diseased and healthy tissue for the carrier molecule. There should be an abundance of the antigen in diseased tissue compared to healthy tissue. Specificity and selectivity to the antigen should be established for the binding agent. Data on activity in source organs from the preclinical dosimetry study needs to be extrapolated to absorbed dose estimates for human target organs. This should be included in your IND application for your first in human study.
Preclinical dosimetry study and preparation before clinical trial
TRACER assists drug developers from the preclinical stage into the clinical phases of drug development. We can perform all preclinical work for your dosimetry study. Please note, not everything in this list is mandatory before you can start your first human study, and other studies may be required based on individual cases. This list is only meant to inform you about common preclinical work.
- Toxicity study (single dose administration on single species (mammalian, both sexes).
- In-vitro studies for target binding.
- In-vitro studies for therapeutic effect (e.g. anti-tumor activity).
- Selection of appropriate species (animal models).
- Animal pharmacology study to determine optimal formulation (radioactive and non-radioactive materials) and the Maximum Tolerated Dose (MTD).
- Usage of the intended route of administration vs intravenous injection.
- Selection of imaging techniques (PET, SPECT, CT, or other).
- Biomarker selection and tissue sampling.
- Organ of interest selection (always bone marrow, kidney, liver, and regulator-defined organs such as brain, intestines, stomach, etc., and study-specific organs, such as eyes or skin).
- Segmentation of regions of interest (whole organs, organ sub-regions, tumors, etc.)
- Biodistribution for evaluation of radioactivity over time.
- Quantitative imaging and analysis plan (include many sampling time points, e.g. from FDA: 5x effective half-lives).
- Calculation of absorption.
- Excretion data should be obtained.
- Obtain micro-scale data (for alpha emitters) on sub-regions of critical organs at micrometer scale (estimates based on cold compounds may also be sufficient).
- Relative organ-mass extrapolation to humans from animal data (your method should be described in your submission).
The preclinical dosimetry study produces a preclinical dosimetry report. This includes the necessary toxicological studies for radiopharmaceuticals to proceed to clinical trials.
5 tips from our dosimetry expert
Tip #1: include the complete decay cascade, including decays and half-lives of daughter isotopes in your animal studies. For example, Actinium-225 and its daughter nuclide Bismuth-213. This is often included in dosimetry software.
Tip #2: consider all emission types of your radionuclide for your study. This is often also included in dosimetry software.
Tip #3: think about your clinical study design, because study design and compound formulation should be similar and consistent. For instance, when you consider pre-treatment to reduce organ uptake (e.g. thyroid protection), this should be similar in your animal study.
Tip #4: include both male and female (organs) in all your studies, unless your diagnostic/treatment or indication is gender specific.
Tip #5: keep in mind the unique aspects of your product. Fast clearance means earlier and more frequent sampling, while slow clearance means more divided sampling.
Clinical dosimetry studies
Clinical dosimetry studies assess the behavior of the radiopharmaceutical in-human. This is a list of dosimetry services TRACER can conduct for you. Keep in mind that regulators would like to see a Proof-of-Concept study (PoC study) prior to the First-in-Human study (FiH study).
- Scanner calibration and image acquisition
- Image reconstruction and quantification
- Drawing of Volume of Interest (VOI) over the target and relevant organs
- Organ dosimetry calculation based on the distribution of Time Integrated Activity (TIA)
- Generation of Time Activity Curves (TAC) / TIA / cumulated activity, and calculation of Regions Of Interest (ROI) (e.g. organ, tumor) and total body dosimetry
- Calculations based on dosimetry software
- Result interpretation and dosimetry analysis
- Blood and urine sampling after each SPECT/CT scan, measurement of radioactivity
- Blood and urine PK analysis (based on radioactivity measurements)
- Guidance for EMA and FDA discussions
Shorter preclinical trajectory for known radionuclides and ligands
When data is already available on your radionuclide and ligand, your preclinical trajectory may be abbreviated. The first in human dose may be based on previous data. Relevant organ-specific data from published animal or human studies should be described. General toxicity studies may be omitted for the radionuclide only (without ligand). A general toxicology study in one species (same as biodistribution and dosimetry study) to evaluate ligand-induced toxicity can be conducted with the non-radioactive pharmaceutical.
Diagnostics and microdosing therapeutics require less preclinical work
The amount of non-clinical supporting data may be lower for diagnostics. Regulators deem the potential for adverse events unlikely when the dose is low enough. Typically, a microdose (≤ 100 µg) – similar to that used in Phase 0. Chronic toxicity studies may be omitted for the non-radioactive pharmaceutical (precursor) when dosing is limited to 2-3 times (with complete wash-out of the radiopharmaceutical before the next administration takes place), a microdose is administered, and the ligand is only for delivery. Drug developers should justify their decision to waive specific preclinical studies.
Genotoxicity, reproductive toxicology, and carcinogenicity studies
Genotoxicity, reproductive toxicology, and carcinogenicity studies may be omitted since the effect is evident for radiopharmaceuticals. Product labeling should include these warnings. When effects related to the above studies have been witnessed during (animal) studies, this should be described. For radiopharmaceuticals information regarding contraception for both men and women should be included. The same applies for lactating women to prevent or limit radiation exposure to the child.
Long term radiotoxicity studies
Long term radiotoxicity studies
Besides toxicity studies for exposure to the ligand, long term toxicity studies may be necessary to investigate the toxicity induced by exposure to radionuclides. Radiation-induced toxicity can lead to delayed or accumulated adverse events. Long term toxicity studies are important for treatments for patients with a long-life expectancy. A long-term radiotoxicity study may be done in a single-species animal study. Microscopic research is necessary for organs with significant pathology findings. A summary of animal distribution studies, human dosimetry studies, and publications on late radiation effects might also be sufficient. The FDA provides guidance on this subject.
Resources on internal radiation dosimetry
Internal radiation dosimetry is calculated for radiopharmaceuticals. Listed below are several useful resources on radionuclide data and decay broadly used in the field.
- Medical Internal Radiation Dosimetry (MIRD)
- Radiation Dose Assessment Resource (RADAR)
- Reports 53 and 80 of the International Commission on Radiological Protection (ICRP)
- International Atomic Energy Agency (IAEA) Dosimetry for Radiopharmaceutical Therapy
- IDAC dosimetry software, freely available at www.idac-dose.org
Dosimetry clinical trial for all types of radionuclide therapy and diagnostics
TRACER can conduct clinical trials for all types of radiotherapy or diagnostics. This includes:
- Radiopharmaceuticals (e.g. topical, oral, or systemic delivery)
- Other internal radiation therapy (e.g. brachytherapy)
- Non-radioactive pharmaceuticals labeled for research
Depending on your compound and indication, your first in-human trial can be Phase 1, but with TRACER you can also benefit from Phase 0 exploratory trials.
Frequently asked questions
Dosimetry can be complex and there is often confusion about the meaning. It is not always clear for dosimetry trials which legal rules apply and which study design should be used. While many answers are specific for each case, we provide a general FAQ on dosimetry. The study of dosimetry comprises the measurement of a radioactive dose delivered and absorbed by organs and tissue. In the medical field, dosimetry studies are part of treatment and are an aspect of clinical trials for new therapies and diagnostics. Dosimetry in radiopharmaceuticals evaluates the safety and efficacy of the radiopharmaceutical. One of the objectives of a dosimetry study is to find the optimal radioactivity dose. Analysis can be done with imaging techniques, such as PET and SPECT, and blood and urine samples. The radioactive dose absorbed by organs and tissue is calculated. In a dosimetry study, calculations are cross-checked with safety parameters such as hematology and blood chemistry measurements for radiotoxicity. Internal dosimetry in nuclear medicine comprises the measurement of radiation in the body from a radiopharmaceutical. Internal dosimetry is studied in clinical trials for novel diagnostics and therapeutics. The biodistribution of a radionuclide is translated to organ and tissue exposure. With the advancements in radiopharmaceuticals for treatment, patient-specific dosimetry has become more important. Part of internal dosimetry is evaluating the distribution and kinetics of a radiopharmaceutical within individual patients. In radiopharmaceuticals, alpha, beta, gamma, and Auger electron-emitters are used. Alpha and beta are used in therapy. Alpha decay is considerably stronger than beta decay but with a much shorter path length. Alpha and beta emitters may also release gamma rays. Gamma is only used for diagnostics. Auger is the least frequently used in clinical trials. The amount of radiation delivered to organs and tissue can cause irreversible damage. In the case of oncology treatment, the killing of cancerous cells is intended. Dosimetry is important in nuclear medicine since it’s a fine line between effective treatment and severe side effects. Treatment would not achieve the desired effect when the dose is too low. But, when dosing is too high, side effects can be considered unacceptable. Preclinical studies and clinical trials aim to evaluate the efficacy and find the optimal dose. MIRD stands for Medical Internal Dose Committee. The MIRD dosimetry method was presented in 1968 by the Society of Nuclear Medicine and serves as a standard in dosimetry. This method is a simplified model of the human body to evaluate radiation absorption in organs and tissues. It uses the physical half-life of the radionuclide and the biological half-life of the radiopharmaceutical in the body. External dosimetry measures the radiation from a source outside the body. Measurements can be achieved with a dosimeter. For example, for personnel working with radioactivity and in radiation oncology. Internal dosimetry measures the radiation from a source inside the body. This is more complex because many factors have an influence on the absorbed dose in organs and tissues. Clinical dosimetry, also known as medical dosimetry, refers to the patient-centered dosimetry tasks. It involves radiation treatment planning, dose calculation, and safety assurance. A clinical physicist or medical dosimetrist works in the clinic and adjusts the dose to specific patients. This is based on, among others: size, body weight, and vulnerability. Radiopharmaceuticals are used in research to study biological processes. Examples are PET and SPECT imaging. TRACER utilizes these imaging methods in clinical trials to assess also non-radiopharmaceutical drugs. By labeling a drug with a radioactive tracer, the biodistribution and pharmacokinetics can be studied. Furthermore, imaging allows us to determine biological processes and characteristics in-vivo. We can identify biomarkers, receptors, genes, activities, and processes. Drug developers can increase their chances of success in clinical trials by an improved understanding of their drug and target. Without going into too much detail, dosimetry studies are based on the source of the radiation and the receiving organs and tissue. Each uses its unit of measurement. There are different types of dosimetry. Below we compare and provide basic information on the commonly used distinctions. The most significant distinctions in types are internal and external dosimetry. Internal dosimetry applies to radiation from radioactive substances administered to a patient or implanted in a tissue of interest, such as a radiopharmaceutical. Measuring the dose from an external source, such as a beam, is named external dosimetry. External dosimetry can be measured with a dosimeter or can be calculated. Dose estimates for internal dosimetry are based on imaging techniques, bioassays, and calculations. These calculations involve biology and physics. Much data is readily available in tables and dosimetry software. Another major difference in dosimetry is active vs passive dosimetry. Active dosimetry, which is sometimes referred to as operational dosimetry, provides real-time information on radiation exposure. Dosimeters also indicate active and passive functionality. Passive dosimetry measures radiation exposure over a period of time. The term “dosimetry” conventionally indicates macrodosimetry, which focuses on the measurement of radiation doses at a macroscopic scale, involving large tissues and whole organs. In contrast, microdosimetry is defined as “microscopic scale dosimetry,” targeting individual cells or subcellular structures. The objective of microdosimetry is to evaluate the particle’s track and lineal energy distribution within cells. This approach provides valuable insights into the radiobiological effects on cells and the mechanisms of radiation damage. To perform microdosimetry detailed information is required on the cellular distribution of the radiopharmaceutical in within the cells. These data is difficult to obtain in humans. Preclinical data is therefore used to make assumptions for dosimetric models for microdosimetry. In clinical trials and treatment, there is a big difference between personalized and standard dosimetry. Where standard treatment uses generalized protocols based on average patient models, personalized treatment is adjusted to the individual patient. In treatment, this can make a big difference. Drug developers should take this distinction into account for their dosimetry trial. Outcomes can deviate significantly, as this study shows. CT imaging can be considered for more precise organ mass calculations. You can also look into the Voxelwise method, as in this study.
What is the study of dosimetry?
What is dosimetry in radiopharmaceuticals?
What is internal dosimetry in nuclear medicine?
What type of radiation is used in radiopharmaceuticals?
Why radiation dosimetry is important especially in the field of nuclear medicine?
What is medical internal radiation dosimetry MIRD method?
What are the basic differences between external dosimetry and internal dosimetry?
What is clinical dosimetry?
What are the applications of radiopharmaceuticals in research?
What is the unit of measurement in dosimetry studies?
What are the types of dosimetry?
Internal vs external dosimetry
Active vs passive dosimetry
Microdosimetry vs macrodosimetry
Personalized vs standard dosimetry
How can TRACER as an early phase CRO assist drug developers?
TRACER can conduct dosimetry studies, but more importantly, TRACER specializes in Phase 0 clinical trials. This first in-human study can be conducted earlier than Phase 1. Already in Phase 0 quantitative imaging is done to locate where the radiopharmaceutical accumulated. It also allows us to measure the emitted radiation and relate that to the activity value. The interval for imaging can be up to several days or even weeks. You can use the data from your phase 0 study to go into subsequent clinical trials better informed.
Although this article has been composed with great care and attention, we cannot guarantee its accuracy. If you have any suggestions or additions to this article, please email info@tracercro.com.
No rights can be derived from this publication. This blog does not make claim or promote ownership to any intellectual property, study information, clinical images, or copyrighted terms wherein.