Antibody fragments are regions of antibodies that can be used for therapeutic or diagnostic use. Fab fragment is the term for the fragment antigen-binding region. The Fab antibody binds to the antigen and can do this without the other regions of the antibody. Fragments can be used in their natural structure or can be engineered. The best example of such an engineered, fab fragment is the single chain variable fragment (scFv). The variable regions of the heavy and light chain are chemically coupled. This technique opened the door to many antibody fragments and antibody platforms. This blog gives you a comprehensive list of common types.
Trending: Antibody fragments
There is high interest in antibody fragments since binding works similarly compared to full-length immunoglobulins. Only the fragments are smaller and therefore offer deep tissue penetration and fast clearance. Fragments are used in many constructs; currently over a hundred have been developed.

Antibody fragments market
The antibody fragments market is rapidly developing. The market share of antibody fragments as therapeutics is expected to grow by around 5% each year. A few examples of antibody fragment types already on the market are:
- Ranibizumab (Fab fragments derived from bevacizumab)
- Brolucizumab (single chain variable fragment (scFv))
- Certolizumab (Fab’ fragment (apostrophe indicates that a part of the hinge is present))
- Caplacizumab (nanobody® (Nb))
- Blinatumomab (bispecific T-cell engager (BiTE))
The antibody fragments meaning for the terms and abbreviations above will be thoroughly explained in this article.
Introduction to antibody fragments
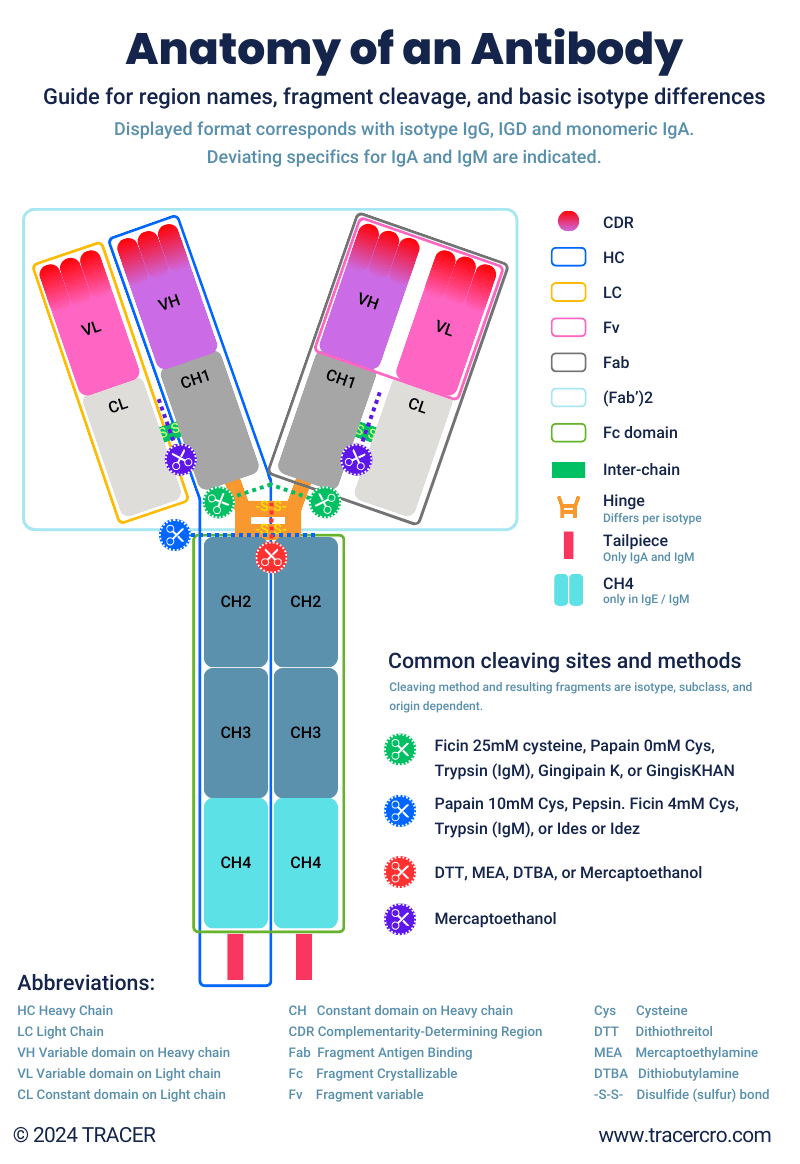
Every conventional antibody has the same characteristic Y-shape as shown in the figure below. The end of each arm enables binding to a specific antigen. This part is commonly used in antibody fragments. There are multiple antibody isotypes, subtypes, and allotypes but all of them consist in general of the same regions. These antibody regions (also referred to as portions, parts, or domains) are connected by hinges that can be split. After splitting, antibody fragments can be derived. These fragments can be used as is or can be used to engineer further constructs.
Hinge and linkers
The hinge region connects the heavy chains of the Fc region with the heavy chain of both arms. Between the heavy and light chains is an inter-chain. Both are disulfide, covalent bonds. These sulfate bonds, often written as -s-s- are cleavable by reducing agents such as the commonly used enzymes papain and pepsin. After cleaving these bonds the antibody is divided into fragments, and bonds can be indicated by -sh (sulfhydryl).
Antibody splitting, the production of antibody fragments
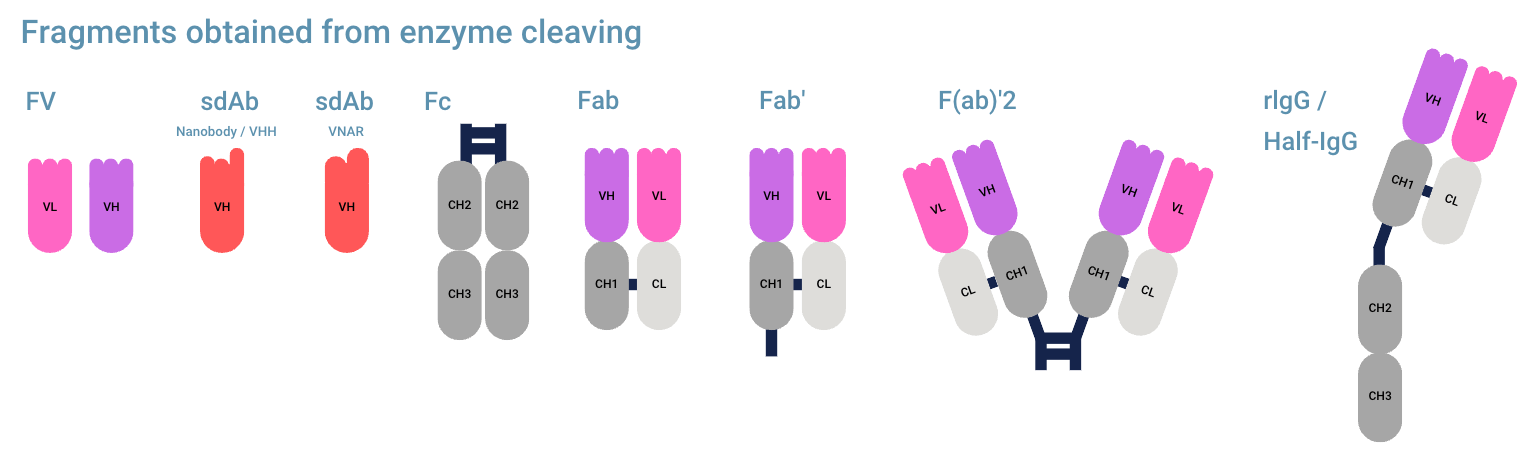
Did you know that there are multiple methods of splitting/cleaving antibodies? Methods can result in different fragments. See figure “Anatomy of an Antibody.” A few common examples of antibody cleaving are:
- Splitting antibodies with the Pepsin enzyme results in one F(ab’)2 fragment and a fragmented Fc domain for each antibody.
- Antibody fragments derived from Papain cleavage are two Fab fragments and an intact Fc domain. One F(ab’)2 fragment can be obtained from Papain which has been activated with cysteine.
- Ficin digestion will break down the Fc domain into numerous fragments, leaving two Fab regions or one F(ab’)2. Ficin is used for mouse monoclonal IgG1.
Important to know, is that not all methods can be used for all antibody isotypes and subclasses. Also, results per origin of antibody can differ.
Antibody fragment production methods
Another method besides enzymatic fragmentation to produce antibody fragments is recombinant DNA technology. Specific antibody fragments can be produced by expressing antibody genes in a suitable host, such as:
- Phage display
- Yeast display
- Bacterial display
- Mammalian cells
In addition to these methods, a few others are:
- Cell-free protein synthesis
- Chemical synthesis
- Protein subunit vaccines
The preferred method is depending on the antibody fragment type and application.
What are the different types of antibody fragments?
There are several types of antibody fragments.
- Natural antibody fragments that are solely derived from cleaving.
- Antibody fragment constructs from re-engineered fragments with linkers.
- Antibody fragment constructs that use parts from multiple different antibodies, other compounds or molecules, or changed antibody structures.
- Artificial antibody mimicking compounds.
Antibody fragments from enzyme cleaving
These antibody fragments can be made by only cleaving the hinge region at specific points.
- Fv (Fragment Variable)
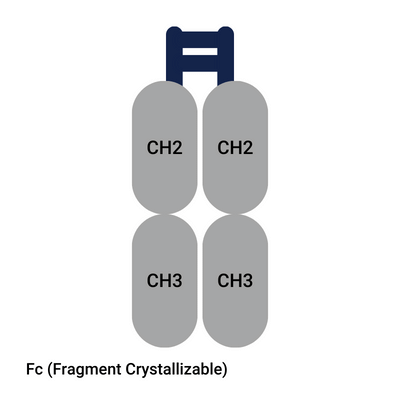
- Fc (Fragment Crystallizable) (antibody tail region)
- Fab (Fragment antigen binding, consists of one constant and one variable domain of both light and heavy chain)
- Fab’ (Fab with part of hinge)
- (Fab’)2 (two Fab regions with hinge)
- rIgG / Half-IgG (only the left or right part of IgG, consisting of one heavy chain (HC) and one light chain (LC))
Other antibody fragments (in general inadvertently) created from cleavage:
- Fd’ (VH+CH1 including a piece of the hinge)
- Fc/2 (half Fc fragment)
- LC (only the light chain)
- pFc (fragmented Fc fragment)
Fab antibody
The Fab fragment interacts with the antigens. For most mammalian antibodies it is of a similar construct. A Fab fragment is made of a heavy and a light chain connected with a disulfide bond and noncovalent interactions such as salt linkages, hydrogen bonds & hydrophobic interactions. Good to know: the heavy chain only antibody lacks the light chain.
Because the Fab region of antibody has high specificity, for drug development the combination of Fab and antigen is essential. When the combination Fab-antigen is found, the Fab fragment can be used for further development. This can be done in full-length immunoglobulin size, Fab2 fragment (both Fab domains), single Fab domain, or any of the other cleavable regions.
FC region of antibody
After cleaving, drug developers get the Fab and Fc region of antibody. The Fc portion of an antibody has a different function than the Fab region. What is the function of Fc region of antibody? The Fc region of antibody binds to cell surface receptors and certain proteins and can trigger the immune response.
FC region IgG effector function
The Fc region of immunoglobulin IgG can interact with the Fc-gamma receptors (FcγRs). This is essential for immune effector responses. These cell surface receptors are found on macrophages, neutrophils, dendritic cells, and natural killer cells. Because of this Fc region function, it plays an important role in drug development. Although the comparison between nanobody® vs antibody is often made, the Fc portion of immunoglobulin enables functions that fragments lacking the fc-domain, like nanobodies®, scFv and Fab fragments cannot. Keep this in mind when you read the next paragraph about nanobodies®.
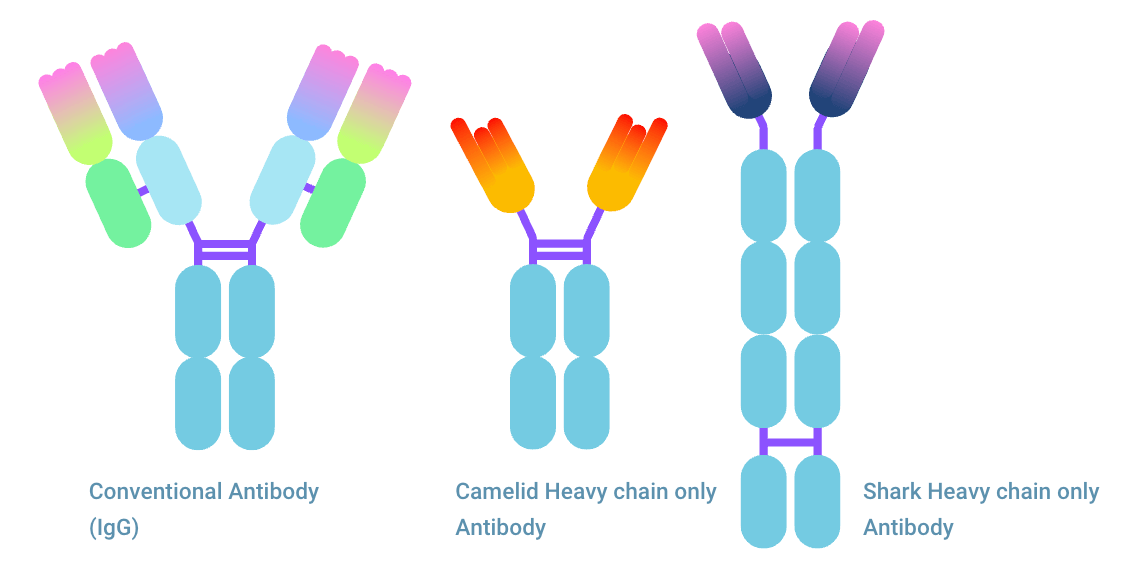
Nanobody® from Heavy chain only antibody
A conventional antibody consists of two heavy chains and two light chains. For antigen binding, Fab fragment needs both the fragment binding region from the heavy and light chain. One single chain FV fragment does not offer the same binding. However, one antibody, the Heavy chain only antibody (HcAb) is significantly different. This antibody lacks light chains and can bind to an antigen as a single domain antibody (sdAb).
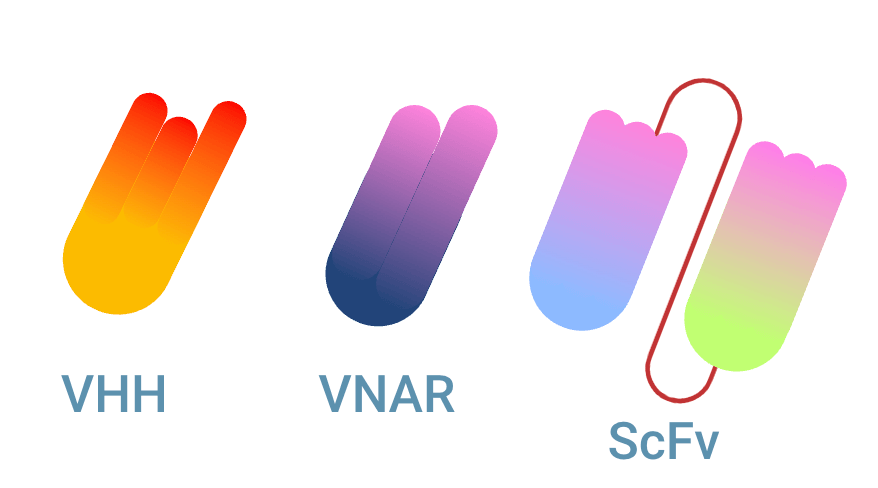
Multiple types of single domain antibody (sdAb)
There are multiple types of single domain antibodies originating from different species. The fragment is named VHH (Variable domain of Heavy chain of Heavy-chain only antibody) when it originates from camelid antibodies. This fragment is also named Nanobody®, a registered trademark of Ablynx N.V. The sdAb is referred to as VNAR (Variable domain of New Antigen Receptor) when it is the variable domain of IgNAR shark antibodies. The scFv fragment can be seen as the equivalent of a sdAb, being the smallest fully capable antigen binding fragment of conventional antibodies.
Antibody fragments with linkers
Not all antibody fragments can be used directly. The derived antibody fragments can be re-engineered with linkers to create many different designs. The most common ones are listed below. When the Fab and Fc region of an antibody are used, it can trigger effector functions. When only parts from the Fab region are used, it can still result in blocking or neutralizing pathogens, antigens, and receptors. It can inhibit pathways without causing an immune response. Full length immunoglobulins can be modified to lack certain effector functions, they are named Fc silenced antibodies.
Linkers
Linkers, made from short sequences of amino acids, connect the N-terminus (amino terminal) and the C-terminus (carboxy-terminal). They serve as a flexible tether and allow fragments to move in an independent flexible manner while maintaining their structure. Antibody fragment engineering makes use of linkers to combine multiple fragments into a new construct.
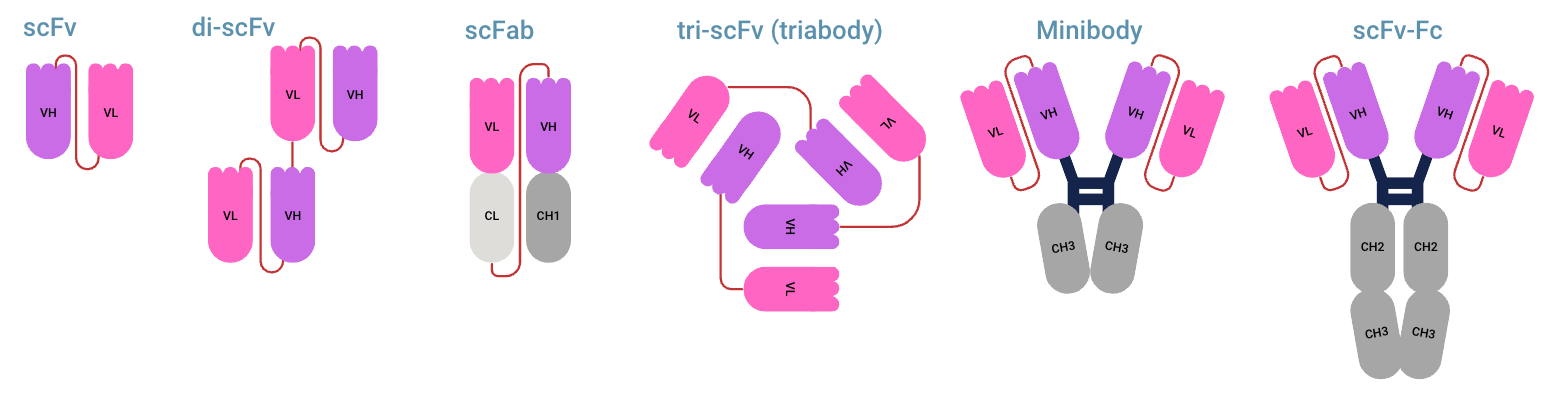
- scFV (single-chain Fragment Variable) (VL and VH fragment)
- di-scFv (sometimes referred to as dscFv or diabody) (divalent scFV, two scFv fragments linked)
- tri-scFv (triabody) (three scFv fragments linked)
- scFab (Single chain Fab) (Fab element, connected by a linker instead of hinge)
- Minibody antibody(two scFv fragments linked to two CH3 regions)
- scFv-Fc (two scFv fragments linked to Fc region, but around 50 kDa lighter than a full-size IgG)
Different antibody fragment categories
Antibody fragments can be subdivided by design and by function. We list the common categories:
- Full-length antibodies symmetrical
- Full-length antibodies asymmetrical
- Antibody fragments with Fc domain
- Antibody fragments without Fc domain / Variable domain only Ab (Antibody)
- Monospecific antibodies
- Bispecific antibodies
- Monovalent antibodies
- Bivalent antibodies
- Multispecific antibodies (trispecific, tetraspecific (four-in-one) etc.)
- Multivalent antibodies (trivalent, tetravalent, pentavalent, hexavalent)
- CH1/CL fusion proteins
- Constant domain-extended bispecific fragments (antigen-binding fragments recombined with (parts of) fc region)
- Appended IgGs (IgG with appended antigen binding fragment(s))
Advantages of antibody fragments over full-size antibodies
As you can see, by linking antibody fragments, new constructs can be created. Antibody fragments can solve problems from full-size antibodies regarding function, biodistribution, and production. Advantages can be regarding a smaller molecular weight, short half-life, increased affinity, valency, and avidity. However, the above list of antibody fragments consists of monovalent, monospecific antibodies. Further engineering allows for multipecific and multivalent antibodies and antibody structures. In addition, new issues with antibody fragments such as a too short half-life, can be solved by fragment design.
Engineered antibody fragments and platforms
Many of these antibody fragments are developed as a platform. Drug developers can use this platform for their antigen binding part. Mostly this is done through a process of in-licensing or out-licensing. In general, this means that the licensor (the company owning the platform) grants the licensee the right to use their platform or technology in their pharmaceutical development and/or product.
List of engineered antibody fragments
Above are just a few schematic examples of monoclonal antibody fragments. There are many engineered antibody fragments. Although the antibody fragment list on this page is extensive, specific technologies or platforms might be missing. This is inevitable since new platforms and technologies are still being developed. In case you miss a specific platform, please let us know.
In general, this list comprehends all major fragment types and many of the platforms and technologies one must know. If an abbreviation has a clear meaning and if we know from a fragment that it is a platform or technology by a company, it’s written behind it.
Download antibody fragment list
Below is a list of common antibody fragments. You can download our Antibody Fragment List with 100+ fragments with images, more information, and relevant links for further research.

Or view first the condensed list without images, short description and (study) links.
Condensed List of common antibody fragment platforms and technologies.
- bi-scFv / bis-scFv (can be designed as bispecific or bivalent scFV)
- bITE (Bicpecific T-cell Engager, platform by Amgen)
- Di-diabody (dimeric antibody fragments)
- Chemically linked (Fab’)2 (bispecific (Fab’)2)
- Tetrabody (technology by Akeso)
- FC-silent (format by AbsoluteAntibody)
- CrossMab Fab (CrossMab is a technology from Roche. It combines two antibodies into one without unwanted formations)
- CrossMab CH1-CL
- CrossMab VH-VL
- Charged CrossMab VH-VL +/-
- Four in One CrossMab CH1-CL
- MAT-Fab (Monovalent Asymmetric Tandem Fab bispecific antibody)
- iBiBody/HBiBody/proBiBody (Platform by Zhejiang Shimai Pharmaceutical Co. Ltd)
- Tandem Forms (Platform by Astellas Pharma Inc)
- ADAPTIR (Ab platform from Aptevo Therapeutics)
- bi-bNAb (bi-specific broadly Neutralizing antibody)
- BiIA-SG
- Kλ Antibody
- BiAbFabL
- DVD-IgG (Dual Variable Domain Immunoglobulin G)
- VCDFc
- VCVFc
- taFab
- DART (Dual Affinity Retargeting Antibody, MacroGenics)
- Trident (Trispecific antibody, MacroGenic)
- XmAb (Ab platform by Amgen)
- BEAT ((Bispecific Engagement by Antibodies based on the T cell receptor) platform from Glenmark Pharma)
- SEEDbody (Strand-Exchange Engineered Domain body)
- DuetMab
- Peptibody (peptide-Fc fusions, platform by Amgen)
- Quadroma
- Knobs in holes cognate light chains (KiH, this technology can prevent the formation of unwanted formations during antibody pairing)
- Knobs in holes common light chains
- TriMab (triple mAb, three antibody-based therapy)
- Triomab (quadrom) (by Fresenius Biotech, TriOn Pharma)
- OAscFab-IgG (One-Arm single-chain Fab-Immunoglobulin Gamma)
- dsFv-IgG (disulfide stabilized Fv-IgG)
- cFAE-IgG1 (controlled Fab-Arm Exchanged IgG1)
- Charged Pair scFv-Fc
- LUZ-Y (format, existing as One-arm and Two-arm (LC LUZ-Y, tethered LUZ-Y, and tethered LUZ-Y with Furin cleavable tethers))
- Tandab (Tandem Diabody, bispecific tetravalent antibody)
- BiKE (Bicpecific Killer-cell Engager)
- TriKE (Tricpecific Killer-cell Engager)
- ANKET (Antibody-based NK cell Engager Therapeutics)
- mFc-VH (monomeric Fc-VH)
- Fcab (Fc antigen binding)
- OrthoFab IgG
- Fv2-Fc
- scFab-dsscFv
- KiH IgG-scFab
- FcΔAdp (by Regeneron)
- ART-Ig (Asymmetric Reengineering Technology-Immunoglobulin, platform by Chugai)
- SMART-Ig (Sequential Monoclonal Antibody Recycling Technology – Immunoglobulin, platform by Chugai)
- BiMab (platform by OncoMed)
- Biclonics (platform by Merus)
- Triclonics (platform by Merus)
- Azymetric (platform by Zymeworks)
- 2:1 TCB (T Cell Bispecific)
- Fab-IgG TDB
- FynomAb (Platform by Covagen AG, part of Johnson & Johnson)
- Two-in-One DAF (Dual Action Fab, technology by Genentech)
- Nb-scFV (sdAb to scFv fragment)
- scFabΔC
- FabΔC
- DiFabody (mulimerisation form of scFabΔC)
- TriFabody (mulimerisation form of scFabΔC)
- DuoBody (platform by Genmab)
- HexaBody (platform by Genmab)
- DuoHexaBody (platform by Genmab)
- HexElect (platform by Genmab)
- Tribody (technology by Biotecnol)
- trAb (Tandem Receptor-Activating Bispecifics)
- TRAb (TSH receptor antibody)
- SyAM (Synthetic Antibody Mimic)
- Fab-PEG (PEGylated Fab’ (polyethylene glycol)
- CODV-IgG / CODV-Fab (Cross-Over-Dual-Variable)
Download this list including images, short description and (study) links
In addition to the list above, there are more fragments and newly invented fragments will be added to the list in the future. Some are named in a manner that you can retrieve information from the name. For instance: “scFv-scFv-scFv” are three scFv fragments linked together.
Learn the language
When you understand the language, you can often understand what an antibody fragment looks like. Below is a short list of words and abbreviations and the corresponding meaning.
Also note that technologies can be adapted into different fragments, meaning formats don’t always look similar while sharing partially the same name. Examples are BEAT, KiH, and CrossMab.
Words and abbreviations definition list
“Charged” indicates that a part of the antibody is charged for the correct pairing of chains. This indicates that the construct is bispecific.
“Knob-in-Hole”, abbreviated to KiH, is a technology for correct pairing, meaning the antibody is probably bispecific and made of two distinct half antibodies. KiH can be used in heavy chain pairing and heavy and light chain pairing.
“Di” indicates two fragments are used.
“Bi”, “Bis”, indicates bispecific antibody.
“Tri” often indicates three binding sites. The construct can be a full length Ig construct with an additional Fab or Fv fragment, or three fragments combined.
“Tand” stands often for “Tandem” meaning two or more fragments are linked next to each other.
“Fab2” or “Fv2” can indicate that there are two binding sites. This is used often when the conventional arms-shaped construct is used. The Fv or Fab fragments don’t necessarily have to be in conventional format, they can also be linked.
“Fv” indicates only the VH and VL fragments are used.
“Fab” indicates the VH+CH1 and VL+CL fragments are used. This can be with an original hinge or in single chain (cs) format.
“IgG” the well-known abbreviation for Immunoglobulin G and in antibody format it often indicates that a full-length IgG antibody is (partly) present.
“Nb” and “VHH” indicate that a nanobody® is present in the construct, where the latter is always of camelid type. The first however is often also of camelid origin, since the only other known nanobody® VNAR, derived from shark, is not yet commonly used. Nb, VHH, and VNAR are never of human origin.
“Sc” means Single chain, it suggests that a linker is used to combine fragments.
“Fc” means that the Fc region of the antibody is used.
“mFc” indicates that a monomeric Fc domain is used, meaning one CH2 and one CH3 domain.
“CH3” often indicates only the CH3 domain of the Fc region is used.
“(H), (L), (HC), (LC)” can be used to indicate where the additional fragment is connected. For example: scFv(LC)-IgG translates to a full-length IgG with added scFv fragments to the light chains.
“IgG2” can have several meanings. In general, it refers to the second antibody subclass of IgG. In antibody fragments, it can also mean two IgG antibodies connected to each other.
“IgG-IgG” can point out that two IgGs are linked together. From this you cannot say for sure if the construct is bispecific and how the antibodies are linked.
“IgG1/IgG2” can refer to a bispecific antibody with one half from IgG1 and the other half from IgG2.
“κ” specifically indicates that a kappa-chain is used, one of the two existing types of light chains in human immunoglobulins. The other type is lambda (λ). Light chains of sigma (σ) type exist in certain animals, but not in humans.
“λ” specifically indicates that a lambda light chain is present in the antibody format.
“dsFv” of “dsscFv” indicates that disulfide bonds are used in Fv or scFv fragments to improve stability.
“Cognate” indicates that the light chain originated from the same source as the heavy chain is used. You see this in bispecific antibodies.
“Common” indicates a bispecific antibody construct with two different heavy chains but a common light chain. Meaning one heavy chain lacks its cognate light chain.
“HCAb” indicates Heavy Chain only antibody. This is commonly not the same as a conventional antibody that lacks the light chain but is an immunoglobulin type on its own. From this type nanobodies® are derived, meaning the format probably contains Nb fragments. HCAb do not occur naturally in humans, only in camelids and sharks.
“T” The presence of a T in the name can indicate a relation to T-cells, for instance: 1 Fab-IgG TDB indicates a “T cell-Dependent Bispecific antibody” and “TCB” is short for “T-Cell Bispecific”, like in the antibody format 2:1 TCB.
Combinations with other proteins or other molecules can be indicated in the name. An example of this:
“scFv-HSA-scFv” is an antibody format made of two scFv fragments linked to one HSA (Human Serum Albumin) protein.
Frequently asked questions about antibody fragments
Antibody fragments are a relatively new topic in life science. As a Clinical Research Organization (CRO) for first in-human clinical trials we are pioneering with drug developers in this field. Therefore, we would like to answer some of the frequently asked questions. Is your question not listed or would you like more information? Don’t hesitate to contact us today.
What are antibody fragments?
Antibody fragments are parts of antibodies. Fragments can be used without further engineering or can be used as building blocks in drug development. Antibody fragments are mostly produced from IgG type antibodies. In particular, the antigen binding part of IgG is used, but more recently the antigen binding fragment of Heavy chain only antibodies have become of interest. They are referred to as nanobodies® and consist, instead of the light chain and heavy chain of conventional antibodies, of only a single domain antigen binding fragment. Another benefit is the ease of production.
What is a single-chain Fragment variable (scFv)?
A single-chain Fragment variable (scFv) is an antigen binding fragment originating from an antibody. By using a linker, the variable fragments of heavy and light chain are coupled. ScFv fragments can be considered the smallest antigen binding format for conventional antibodies. They are slightly larger than nanobodies® (single domain antibodies (sdAb)). Read the complete comparison between scFv vs sdAb.
What is the difference between Fab and scFv antibody?
The Fab region of an antibody contains the upper part of the heavy chain and the complete light chain. Both are made up of a constant domain (CH1 and CL) and the variable domains (VH and VL). The difference between Fab and scFv lies in this constant part. For a scFv domain, the CH1 and CL are removed. Since a conventional antibody needs both the variable region from the heavy chain and the light chain, these two are reconnected with a linker. This fragment that is engineered, is named scFv fragment. scFv stands for single-chain Fragment variable.
How are antibody fragments produced?
This is the short answer to this question, without going too deep into the methods. Antibodies are produced in the body by B-cells. Therefore, the start of antibody development is by immunization of animals. Isolated B-cells that produce the desired antibody are then fused with myeloma cells. These are named hybridomas and they can produce the right monoclonal antibody in large quantities. To create antibody fragments, full-length antibodies can be cleaved by enzymes. Another method is genetic engineering, where through DNA encoding antibody fragments are produced by host cells (e.g., bacteria, yeast, or mammalian cells). Yeast and mammalian cell lines, e.g. Chinese Hamster Ovary (CHO), and Human Embryonic Kidney (HEK) are also able to produce full-length antibodies.
What are the Fab and Fc fragments of antibodies?
What are the Fab and Fc fragments of antibodies? See figure “Anatomy of an Antibody”. Antibodies are Y-shaped. The two upper arms of the Y are named Fab fragments since they bind to the antigen. Fab stands for Fragment Antigen Binding. Both Fab fragments are referred to as F(ab)2. The lower part of the Y, the stick so to speak, is the Fc fragment of antibody. This Fragment Crystallizable domain does not have the ability to bind to an antigen but has an effector function. The Fc region of antibody binds to cell receptors and proteins. It allows for the triggering of an immune response. When the antibody fragment is produced with Papain, it leaves the drug developers with two fab fragments and one Fc fragment. When Pepsin is used, it splits the antibody into one F(ab)2 fragment, and a fragmented Fc fragment (pFc).
What are the disadvantages of scFv?
The scFv fragment is a widely used antibody fragment. It is small in size and can be engineered as multi-specific. This can be beneficial, although there are also disadvantages of scFv. Please note that some disadvantages can be overcome by engineering, don’t occur in all cases or can be an advantage in certain scenarios.
ScFv serum half-life
The first disadvantage is the lower serum half-life of <1 day compared to 3 weeks for full-size immunoglobulins. For imaging and targeted radionuclide therapy this can be an advantage.
scFv affinity and stability
The second is the decreased affinity since scFv are monovalent, while full-length Ig’s are bivalent. With scFv there is a higher risk of sub-optimal stability and aggregation. This is due to an increased sensitivity to heat denaturation and increased aggregation under thermal stress. Some disadvantages can be overcome through engineering.
For more on this, read: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7546209
What is the structure of a diabody?
A diabody exists of two scFv on complimentary polypeptide chains. This construct provides in general a higher affinity than singular scFvs. The diabody consists of two scFvs, with in total four fragments (2x VH and 2x VL). To form diabodies, scFVs are dimerized by linker peptides that are too short for the Fv regions to fold together. Diabodies are also named di-scFvs (divalent). The way the linker is constructed differs diabodies from other similar formats, like bi-scFv (bivalent) and tandem scFvs.
Are all names for antibody platforms unique?
Please note that depending on the source, you might find different constructs with the same or similar names. For instance, diabody can be of bivalent or bispecific formations. The same applies to trivalent and trispecific triabodies and tetravalent and tetraspecific tetrabodies. Tetrabody can refer to a combination of four scFv domains or to Akeso’s tetravalent bispecific antibodies. Akeso Tetrabody is very different, it consists of a full-length immunoglobulin with two additional scFv domains linked to the Fc-domain. Furthermore, a unique construct can be referred to under different names. One example of this is Fab-scFv / Fab-Fv / Bibody.
Clinical trials for antibody fragments
After drug discovery, the phases of drug development begin. Choosing the fragment design influences Biodistribution (BD) and Pharmacokinetics (PK). It can be challenging to reproduce and establish results from the preclinical phase in clinical trials. At TRACER we specialize in first-in-human clinical trials. Often our Phase 0 imaging trials in-human can replace extensive animal research. This can decrease time and costs in drug development and provide drug developers with visual BD/PK data. Contact TRACER today to discuss the possibilities for your antibody (fragment) or compound.
Download Antibody Fragment List including images, short description and (study) links.
Although this article has been composed with great care and attention, we cannot guarantee its accuracy. If you have any suggestions or additions to this article, please email info@tracercro.com.
No rights can be derived from this publication. This blog does not make claim or promote ownership to any intellectual property, study information, clinical images, or copyrighted terms wherein.
Abbreviations
| -S-S- | Disulfide (sulfur )bond |
| -sh | sulfhydryl |
| (Fab’)2 | Both Fab with hinge |
| Ab | Antibody |
| ANKET | Antibody-based NK cell Engager Therapeutics |
| ART-Ig | Asymmetric Reengineering Technology Immunoglobulin |
| BD | Biodistribution |
| BEAT | Bispecific Engagement by Antibodies based on the T cell receptor |
| bi(s)-scFv | bispecific or bivalent scFV |
| BiTE | Bispecific T-cell engager |
| C terminus | Carboxyl-terminus |
| CDR | Complementarity-Determining Region |
| cFAE | controlled Fab-Arm Exchanged |
| CH | Constant domain on Heavy chain |
| CHO | Chinese Hamster Ovary |
| CL | Constant domain on Light chain |
| CODV | Cross-Over-Dual-Variable |
| CPAB | Cell-Penetrating Alphabodies |
| CRO | Clinical Research Organization |
| Cys | Cysteine |
| DAF | Dual Action Fab |
| DARPin | DARPin (Designed Ankyrin Repeat Proteins |
| DART | DART Dual Affinity Retargeting Antibody |
| di-scFv | divalent scFV |
| dscFv | divalent scFV |
| dsFv-IgG | disulfide stabilized Fv-IgG |
| DTBA | Dithiobutylamine |
| DTT | Dithiothreitol |
| DVD-IgG | Dual Variable Domain Immunoglobulin G |
| Fab | Fragment Antigen Binding |
| Fab’ | Fab with hinge |
| Fc | Fragment Crystallizable |
| Fc/2 | half Fc fragment |
| Fcab | Fc antigen binding |
| FcγR | Fc-gamma receptor |
| Fd’ | VH+CH1 including a piece of the hinge |
| Fv | Fragment variable |
| HC | Heavy Chain |
| HEK | Human Embryonic Kidney |
| HSA | Human Serum Albumin |
| IgA | Immunoglobulin Alpha |
| IgD | Immunoglobulin Delta |
| IgE | Immunoglobulin Epsilon |
| IgG | Immunoglobulin Gamma |
| IgM | Immunoglobulin Mu (M meaning Macro) |
| IgNAR | Immunoglobulin New Antigen Receptor |
| KiH | Knob in Hole / Knob into Hole |
| LC | Light Chain |
| MAT-Fab | Monovalent Asymmetric Tandem Fab |
| MEA | Mercaptoethylamine |
| mFc-VH | monomeric Fc-VH |
| N terminus | NH2-terminus |
| Nb | Nanobody® |
| OAscFab-IgG | One-Arm single-chain Fab Immunoglobulin Gamma) |
| Ortho | Orthogonal |
| PEG | Polyethylene Glycol |
| pFc | fragmented Fc fragment |
| PK | Pharmacokinetics |
| rIgG | reduced IgG |
| scFab | single chain Fab |
| scFv | single chain variable fragment |
| sdAb | single domain Antibody |
| SEEDbody | Strand-Exchange Engineered Domain body |
| SMART-Ig | Sequential Monoclonal Antibody Recycling Technology Immunoglobulin |
| SyAM | Synthetic Antibody Mimic |
| Tand | Tandem |
| TCB | T Cell Bispecific |
| TDB | T cell Dependent Bispecific |
| trAb | tandem Receptor-Activating Bispecifics |
| TriKE | Tricpecific Killer-cell Engager |
| TSH | Thyroid-Stimulating Hormone |
| VH | Variable domain on Heavy chain |
| VHH | Variable domain of Heavy chain only antibody |
| VL | Variable domain on Light chain |
| VNAR | Variable domain of New Antigen Receptor |
| κ | Kappa |
| λ | Lambda |