A bridging trial provides missing (clinical) data without the need to redo initial studies. The term bridging study can be used in two distinct scenarios. Bridging studies commonly refer to studies that fill a gap in regional or regulatory differences and evaluate the comparability in safety and efficacy in ethnically different groups. It may also be used for a study to supplement missing data, for example, in literature, or a toxicity bridging study providing missing preclinical toxicology data. This blog describes the first, bridging trials to obtain market approval in different regions. At the end, we shortly elaborate on other types of bridging studies.
What is a bridging trial? (ICH E5 guideline)
Simply said, it is a study to obtain data on efficacy, safety, dosage, and dose regimens in a population different from the original clinical trials. Regulation is harmonized under the ICH E5 guideline. This states that the study design should characterize ethnic differences (a.o. ADME and food drug and drug-drug interaction) for new medications without unnecessary duplication of earlier studies. For drug developers, bridging trials offer an opportunity for fast approval in new markets.
Bridging trial for regional approval
A bridging trial to gap regional or regulatory differences offers a fast and reliable pathway to reach new populations in new regions. Its goal is to make new therapies faster available to patients globally. A bridging study can be initiated for both a drug already on the market or still in development. Bridging trials are used for regional approval, but its ideology, like regulatory compliance and participant selection, can be used for global drug development.
Start your global development program in the early phase
The ICH underlines that for a global development program the definition and characterization of pharmacokinetics, pharmacodynamics, and dose-response (see ICH E4) should take place early in the clinical phase. At TRACER we suggest that by incorporating this into human exploratory trials, the need and nature of future bridging studies can already be determined. With a smart clinical trial design, you can assess the influence of ethnic factors on safety and efficacy. This may lead to fast acceptance in other regions with minimal additional work and delay. This also allows for the inclusion of representative populations fór the regions (not necessarily ín those regions) where market authorization will be requested.
When is a bridging trial needed?
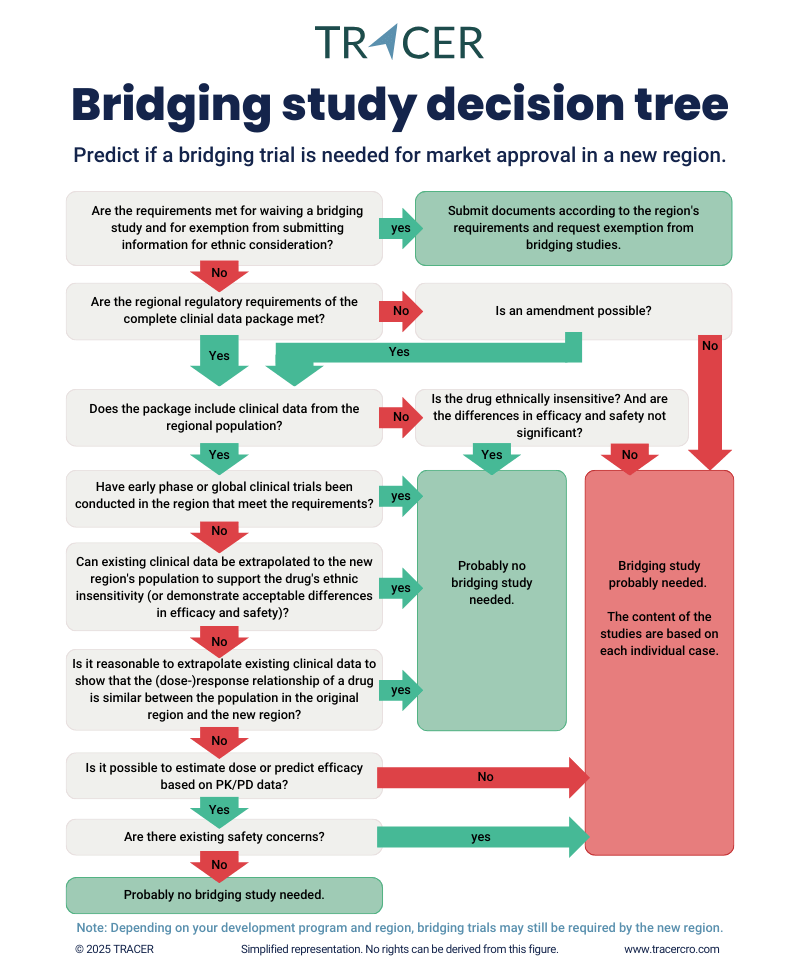
Most importantly, a bridging trial is needed to study how ethnical differences may influence the drug’s behavior in humans. A bridging study takes into account intrinsic (patient’s genetics or physiology and pathological conditions) and extrinsic (patient’s culture and environment) factors. Bridging may also be needed when there are differences in regulatory standards. To find out if a bridging trial is needed, it should first of all be clear if a drug is prone to ethnical differences. Secondly, it should be determined if existing data can be extrapolated and if regulatory standards (a.o. requirements on clinical trials) are met.
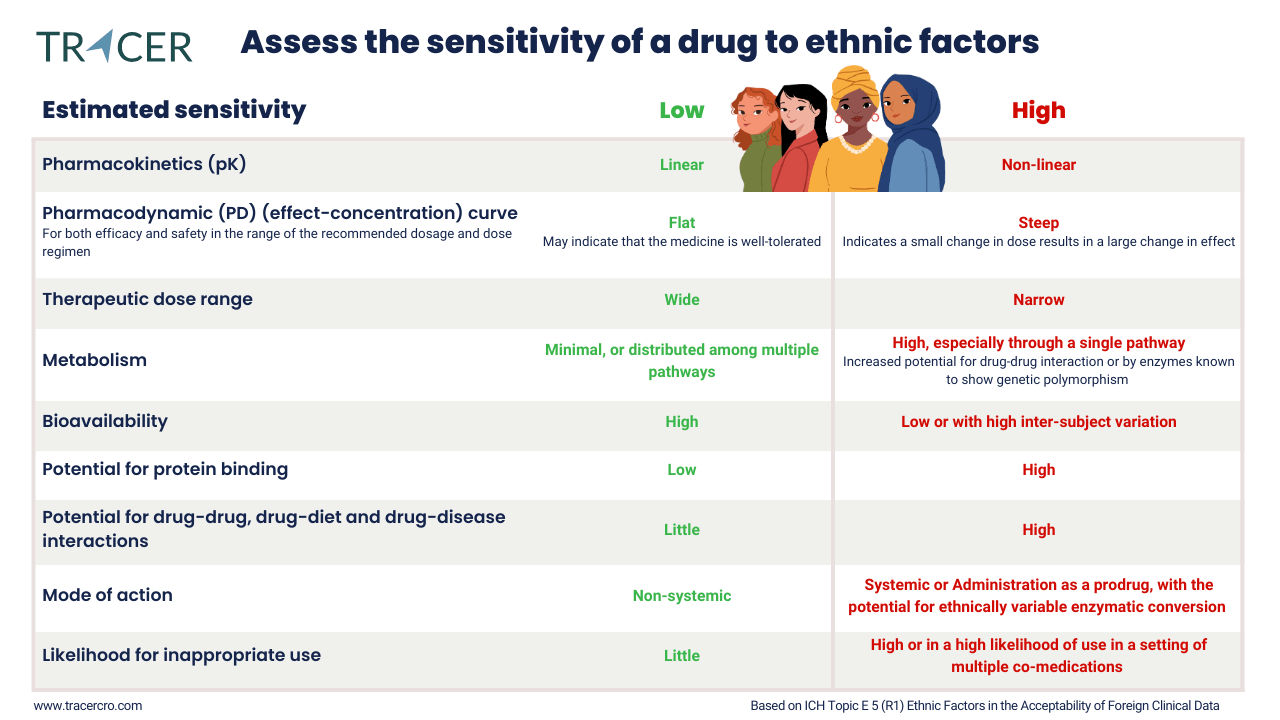
What bridging studies are needed?
Ultimately, this depends on the regulations in the new region. A few examples of bridging studies that may be requested:
- Pharmacokintic study (PK bridging study)
- Controlled pharmacodynamic study (PD bridging study)
- Dose response study
- Safety study
- A single confirmatory trial to demonstrate the ability to extrapolate existing data
- Controlled clinical trial with a clinical endpoint from the original trial, standard clinical endpoint, or a validated surrogate endpoint
Diminish the need for a bridging study
According to ICH standards, a new market should only request bridging studies that are needed to extrapolate the existing data and meet the region’s regulatory standards. You can minimize the need for additional studies by already incorporating factors for other regions in your primary clinical development. Factors such as complying with regulatory requirements and including participants ethnically relevant for that region.
Tips for global drug development:
- Make sure your clinical trial meets regulatory requirements among all ICH regions (e.g. controls, endpoints, GCP, use of accepted medical and diagnostic definitions).
- Include populations from different ethnical groups (check requirements for validity, such as number of generations, gender, age, and body type) and subsets (such as patients with genetic diseases, hepatic dysfunction, renal insufficiency, cardiovascular conditions, and other relevant diseases).
- Pay close attention to dosage, dose regimen, ADME, drug-food and drug-drug interaction, and metabolism (and explanation of ethnical differences, like clearance by enzymes or genetic polymorphism).
- Assess sensitivity to ethnic factors and influence on safety and efficacy
- Analyse dose-response curve and therapeutic dose range.
- Conduct research on other similar medicines and drug classes in the new regions.
How can TRACER, an imaging CRO, help in global drug development?
TRACER specializes in early-phase clinical trials and nuclear and fluorescent imaging. This supports you as a (global) drug developer in two ways.
We conduct first in-patient studies, also known as Phase 0, early Phase 1, or exploratory trials. In such a trial, with around 10-30 patients, we assess the biodistribution and pharmacokinetics of your drug. By doing so in a diverse population, you can address ethnical differences early in your development program.
By using fluorescent and nuclear imaging, you can, even at cell level, determine the presence of your study drug. When using a radionuclide for labeling the compound, small molecule, protein, or any other type, dosimetry calculations can provide an early assessment of efficacy and safety. For questions on how to design a Phase 0 study, please contact us.
Difference in ethnic groups may lead to different imaging outcomes
Good to know, ethnic differences may lead to different imaging results. This study[1] shows a table (table 2) with the differences in amyloid PET positivity for various ethnic groups.
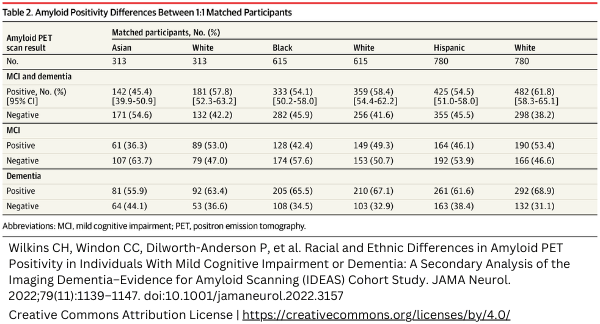
It also highlights the importance of diversity in clinical trial inclusion. For the FDA approved drug, Aducanumab, the percentage of Asian, Black, and Hispanic participants was respectively 10%, 1%, and 3,4% in Phase 3 clinical trials. Another study [2] takes differences in imaging based on race even further, showing AI could be used to identify race based on medical images alone. The study reveals the subtle differences from ethnicity, often unrecognizable by humans. By using imaging in early Phase clinical trials, you can identify ethnical differences in patient groups that might be important to your drug’s behavior.
Bridging studies as part of multi-regional clinical trials
Bridging studies are available for drugs already approved in one or more markets and also for drugs currently still in development. Instead of conducting a bridging trial after market approval, it can be implemented in your current drug development plan. A global development approach can result in simultaneous market approval in different regions. A multi-regional clinical trial is not always necessary, as long as the necessary ethnically relevant groups are included.
Market approval in new markets
Market approval is based on your Clinical Data Package (CDP), together with, if needed, the Bridging Data Package (BDP). With a positive outcome, drug developers are one step closer to market authorization in new parts of the world. This means patients with different ethnicities can be treated safely. Seeking approval in new regions makes medicines available to more patients and can increase the valuation of a drug development program, so it’s a win-win! Read ICH E5 Ethnic Factors in the Acceptability of Foreign Clinical Data [3] for more information.
Common Technical Document – ICH CTD
The common technical document, CTD for short, is used in regulatory review for quality, safety, and efficacy data. Because only module 1 is region-specific, the other modules are the same for all regions. When starting your drug development program, work according to ICH requirements. This ensures compliance with regions developing their regulations under ICH guidance and makes a global drug development program easier.
Other types of bridging studies
You now know what the term bridging study generally refers to; however, the term “bridging” is also used in other scenarios. It can mean a study to supplement missing data, for example, in literature, to support well-established medicinal use. When changes in a study occur, a bridging study may also be needed. For example, in preclinical studies, when switching rodent strains. Or in the clinical stage if changes to the drug or procedure are made. As you will understand from the examples below, the term is used in combination with a variety of studies, all meaning something slightly different.
Examples of other types of bridging studies
- Toxicity bridging study to amend missing preclinical toxicology data or to compare impurities of a GMP-grade substance to earlier GLP-grade material.
- Bioequivalence studies for bridging already conducted (pre)clinical trials of a drug with a generic copy or with several distinct formulations of the same drug.
- Comparability bridging studies for comparison of a drug’s quality, safety, and efficacy after changes in manufacturing.
- Comparability studies to study similarity between two drug products.
- When the drug already has a New Drug Application (NDA), changes in formulation, indications, dosage, manufacturing, or, for example, to make any changes to the label should be submitted as a supplement NDA (sNDA).
- Bridging bioequivalence studies to compare a copy of an approved drug – similar to other bioequivalence studies – but in a region other than the original drug.
- Non-inferiority trials to compare a new treatment with the treatment already available.
Frequently asked questions
To test your knowledge obtained from this blog, let’s answer some frequently asked questions.
What is a bridging study?
According to the ICH, a bridging study is a study that compares a drug in different ethnic groups. More generally, the term bridging study can also be used for different types of studies, which simply means bridging a gap in the data or bridging previous studies to a new situation.
What is a bridging trial?
A bridging trial is a clinical trial designed to bridge earlier obtained data to a new situation. The best example is when the safety and efficacy of a drug has already been proven in one population, and now the same must be proven in an ethnically different group.
What are the main types of bridging trials?
- A study to compare ethnic properties for a specific drug
- A study after (small) changes in manufacturing, formulation, study design, indication, dosage, or changes on the label.
All bridging trials have a similar goal: minimize additional studies by demonstrating similarity to what has already been studied. The contents of such bridging trials should be determined depending on the situation.
Is a bridging study for one single region?
No, you can include bridging studies for multiple regions or incorporate them as part of a global drug development program. So, think outside the box and aim to help the patient, no matter where that patient is.
What is bridging in pharma?
In pharma, it is not uncommon that a drug development program evolves during its existence. In order to compare the effect of the changes with the previous data, bridging might be necessary. Changes to the drug, usage, and procedures, or changes in population (ethnical differences or different patient populations) should be studied carefully. So, after such a change, it is important to evaluate potential changes in safety, efficacy, dosage, dose regimen, ADME, drug-food and drug-drug interaction, metabolism, etc.
What is a pharmacokinetic bridging study?
When changes to a drug development program occur, this may affect pharmacokinetics. For example, when changing the method of administration or changing the compound itself, it should be assessed if the concentration after administration, in blood, for example, is still the same. A pharmacokinetic bridging study asses such changes. In global drug development, a new region for which a representative population was not included in previous clinical trials, routinely requests such a study before market approval can be granted.
Abbreviations
| ICH | International Conference on Harmonization |
| ADME | Absorption, Distribution, Metabolism and Excretion |
| CDP | Clinical Data Package |
| BDP | Bridging Data Package |
| GCP | Good Clinical Practice |
| NDA | New Drug Application |
| sNDA | supplement NDA |
| PET | Positron Emission Tomography |
Citations
Although this article has been composed with great care and attention, we cannot guarantee its accuracy. If you have any suggestions or additions to this article, please email info@tracercro.com.
No rights can be derived from this publication. This blog does not make claim or promote ownership to any intellectual property, study information, clinical images, or copyrighted terms wherein.