Looking for a clinical trial protocol example or template? Keep in mind that different regulatory organizations use other templates. Also, the content of the clinical trial protocol is highly specific to each study. In this blog, you have access to the links to the clinical trial protocol template from several regulatory bodies. After reading, you will understand how to find a relevant clinical protocol example so you can write your own. If you need advice or writing or reviewing of your study protocol, you can schedule a meeting with our regulatory expert and physician-scientist to ask specific questions you might have. This meeting takes around 30 minutes and is completely free of charge.
Contact TRACER
Download: clinical trial protocol template Word
There are multiple clinical trial protocol templates available to download. Which one is right for your trial depends on the country of regulatory body you plan to submit your protocol to for approval. Below, you can find two Dutch organizations and their respective templates for clinical trials in the Netherlands. The template of the EMA for EU clinical trials is also listed.
- You can download a clinical trial protocol template in Word from the National Dutch Ethics Committee, the CCMO, website.
- You can also find a template on the websites of the 13 accredited Medical Research Ethics Committees (MRECs), such as METc Groningen.
- Or on the website of the EMA.
Things to know for safe downloading clinical trial protocol templates
It is logical to use the template of the reviewing regulatory body. In most cases, your study will be evaluated by the regional Ethical Committee. Otherwise known as EC, METc or IRB. Only in certain cases the CCMO will evaluate your study. These are studies with specific subjects, ethical aspects, and research with scarce expertise. The criteria can be found on the ethics committee website. Keep in mind that there can be updated versions of templates, it is common to use the latest version. Check on the National Ethics Committee which template is the most recent and compare it with regional templates.
Clinical trial protocol example PDF
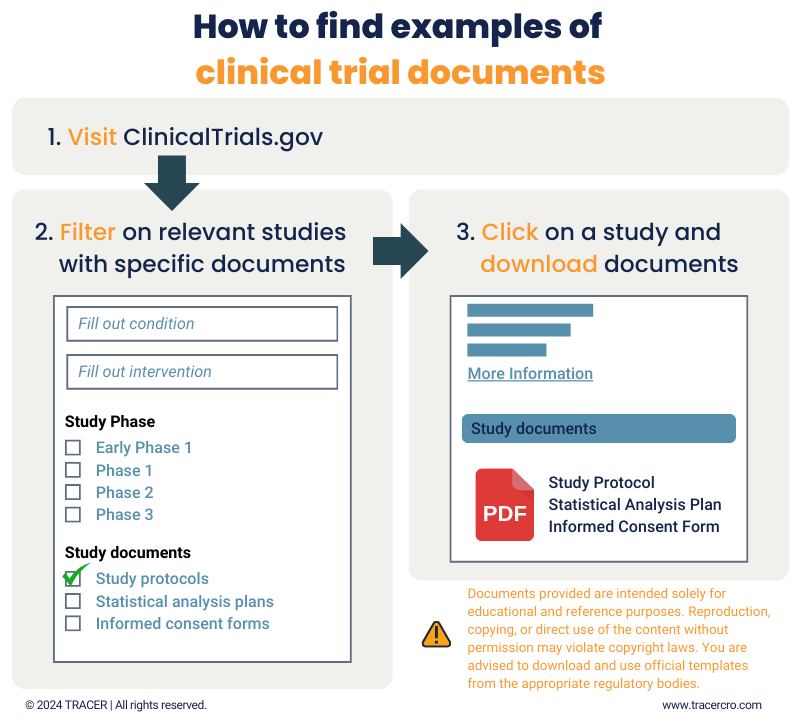
Sometimes, it can be easy to have a clinical trial protocol example PDF to look at. Not for all studies, but for some you can find the clinical trial protocol as an appendix on its ClinicalTrials.gov page.
When searching for similar studies to yours, you can turn on the filter for “study protocols” under the section study documents. Especially when its your first time writing a study protocol, it can be instructive to read example trial protocols first.
When writing a clinical trial protocol, ask yourself, is it justified for me to do something, and do I make clear why?
Anne-Fleur Verhaar, MD
Tips for clinical trial protocol writing
A well-written, and therefore, clear clinical protocol can save time in obtaining regulatory approval, as it may lead to less or even no Request for Information (RFI) from the regulatory body. Furthermore, it can improve the quality of the study. In other words, a well-written clinical research protocol can speed up the approval process, optimize study conduct, and minimize the risk of discrepancies in data due to ambiguities.
Tips from our medical writers
- To write the protocol, you need data from earlier (non-clinical) studies. You can use the IB for information, the IMPD is not necessary to already have available.
- Add a clear schedule of assessments and study procedures.
- Add a flow chart of the study design.
- Your risk-benefit analysis should be clear.
- When writing, think like the ethics committee that will review your research. Consider why you are doing what, and what the justification is for the procedures you propose for subjects.
Frequently asked questions
We hope you’ve learned more about writing clinical protocols from reading this blog and that the links to the templates and clinical research protocol examples will be beneficial to you. We end this article by answering some of the frequently asked questions on this topic. If you have another question or you want an experienced reader to review your protocol, contact us. In short, clinical trial protocols outline the study objectives, procedures, and analytical methods. The document is evaluated by an ethical committee to ensure the safety for participants and compliance with ethical standards. Protocols ensure the quality of research by standardizing the conduct of research between different sites and practitioners. They promote transparency and reproducibility in research, improving the quality and reliability of the data. At TRACER your clinical trial protocol will be written by our physician-scientists. You, as a drug developer, can also write your own protocol, hire a medical writer, or have a consultant write this document for you. In those cases, we can review your protocol accordingly. Sometimes certain medical specialists are needed to write parts of your clinical research protocol. For this, TRACER relies on its vast network. The main objectives of protocol writing in clinical research, is to lower the chance of RFIs since this will cost you valuable time, and to make sure you have a clear guidance for study execution. On the website of ClinicalTrials.gov you can filter for studies that have published their clinical protocol. These can serve as a clinical trial protocol example. This can be useful, but we would recommend first downloading the clinical trial protocol template. The template also includes notes on the information that should be filled out. This is often sufficient to write your document. Yes, when you look at Phase 1 or Phase 3 clinical trial protocol examples, you’ll see differences. However, the template is the same. There is no specific phase 1 clinical trial protocol template for clinical trials in the Netherlands, for example. You include several analysis sets in your clinical trial protocol. These analysis sets include the per protocol set, safety set, evaluable analysis set, and full analysis set. Each set describes the inclusion and exclusion criteria for study analysis. Per protocol analysis means that the analysis is only performed on the set of subjects who adhered to the protocol. Depending on the type of clinical study, ethical commissions accept the use of per protocol analysis or, for instance, require intention to treat analysis instead. A more comprehensive statistical analysis plan for the clinical trial will be included in a different document than the protocol: the statistical analysis plan.
What are clinical trial protocols?
Who writes protocols for clinical trials?
Where can I find clinical trial protocols?
Is the study protocol dependent on the clinical phase of the study?
What is per protocol analysis?
What’s next?
- We recommend you download the clinical study protocol template from the relevant website of your ethical commission.
- If you are inexperienced in protocol writing, read a study protocol example to understand the contents.
- Discuss your study protocol and clinical trial with TRACER, especially before submitting, so we can do a final check to reduce the chance of RFIs and therefore time loss.