You’ve probably heard of 3R animal research where the goal is to Reduce Refine Replace animal testing. The 3Rs animal testing presents a clear objective but does not specify how this can be achieved. So, how can we in drug development reduce refine replace animal testing? What scientific, technological, and regulatory advancements have become available to apply 3R strategies? TRACER can assist you with this. Implementing the 3Rs with our Phase 0 approach can save time, reduce costs, and increase success rates for drug development. Contact TRACER for advice on how the 3R principles may optimize your preclinical and early clinical studies.
3Rs animal testing
Over 50 years ago, the 3 R of animal research was introduced. What is the 3Rs animal model? The framework for 3R laboratory animals consists of Replacement, Reduction and Refinement. The table below explains 3 R’s animal testing with the scope and examples of how to implement it.
| 3R | Goal | Example Methods |
| Replace | Replace animal testing with alternatives. | Early in-human testing, advanced in-vitro models (organoids, organs-on-a-chip), new in-silico models (AI, simulations), ex-vivo testing. |
| Reduce | Minimize the number of animals used for testing. | Improve study design, integrate multiple preclinical studies, use statistical methods (power analysis), non-invasive imaging, data sharing to avoid redundancy. |
| Refine | Improve the life of animals in laboratories. | High-quality and ethical standards, pain and stress reduction measures (e.g., anesthetics), less invasive techniques, improved housing, behavioral and social enrichment. |
Send the 3R table (PDF) to my inbox
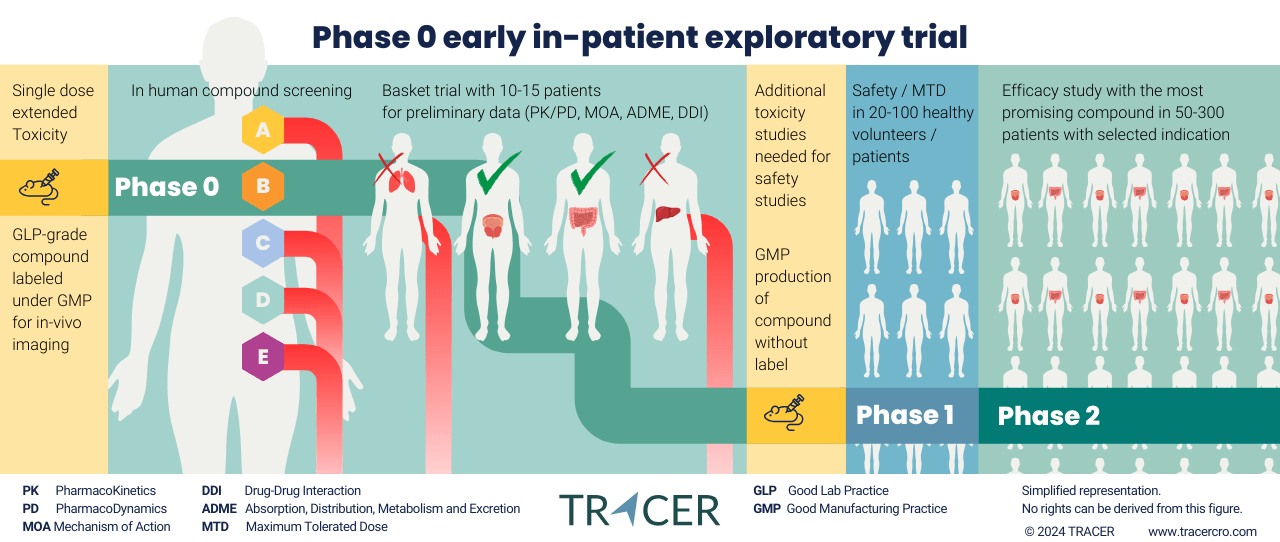
What are Phase 0 clinical trials?
Phase 0 trials are early-stage, first-in-human clinical trials fully in line with the 3R principle for animal research. Phase 0 studies are aimed at assessing pharmacokinetics and pharmacodynamics in patients with a microdose of the compound. A small, subtherapeutic dose of a study drug labeled for imaging is administered to human subjects. Medical imaging allows researchers to gather early data with minimal animal use and human subjects eliminate the risk of interspecies variations. Allowing drug developers to advance promising drug candidates more efficiently into further clinical trials.
3R animal research examples from TRACER
TRACER is a preclinical and early clinical contract research organisation. This position allows us, or perhaps even obliges us, to apply 3R rules in animal research. TRACER’s solutions allow you as a drug developer to take your 3Rs in animal research a step further. Our way of working mainly replaces and reduces the use of animals for testing. We only work with laboratories working according to Refinement, the final 3R principle animal research. To get a better understanding of TRACER’s impact, let’s take a look at 3R animal research examples.
Examples of replacement in animal research
The most effective, and therefore, important R rule in animal research is replacement. TRACER specializes in Phase 0 drug development, a fast translational clinical trial to translate preclinical results (laboratory, animal studies) into the clinic (study with human subjects). Phase 0 first in-human clinical trial can already be conducted after a single-dose extended toxicity study. Safety for participants is ensured by administering only a small amount, also known as microdose, of the study drug. Extensive animal research can be postponed or even omitted until you have results from Phase 0. This is in line with the ICH – including FDA and EMA – guidelines.
Other options for replacement are:
- Advanced in-vitro methods such as tissue engineering, organoids, and organ-on-a-chip.
- In-silico methods, meaning computational models such as AI and simulations of biological systems used to accurately predict behavior in-human.
- Ex-vivo testing on organs or tissues outside the body and without live animals.
In accordance with the 3Rs principles on animal use (Directive 2010/63/EU), a scientifically satisfactory method or testing strategy, not entailing the use of live animals, should be used wherever possible.
EMA Guideline
Examples of reduction in animal research
Phase 0 reduces the use of animals in research significantly by moving early in-human. More methods can also lead to a reduction in animal research.
- Improve the preclinical study design to reduce the number of animals.
- Choose advanced statistical and analytical methods to determine the minimum number needed for statistical significance. This applies especially to toxicity studies.
- Integrate multiple preclinical objectives into one study to reduce the number of animals in preclinical testing.
- Improve drug candidate selection for future clinical trials by in-human screening. This can increase the success rate and reduce the number of failed clinical trials.
- In-vivo, non-invasive imaging can be used to reduce the number of animals sacrificed at multiple steps in testing.
- Derive information from existing, similar studies that may omit specific animal studies.
- If possible: Reuse animals for other studies or rehabilitate animals after a study is completed. (This is also refered to as the 4th and 5th R in animal research).
Example of refinement in animal research
Refinement also benefits from non-invasive imaging, decreasing pain and harm to the animals. Refinement further includes high-quality housing units and improved living conditions for animals. Refinement also depends on highly-trained personnel and adequate protocols. TRACER only cooperates with laboratories that meet the highest quality standards.
In conclusion: reduce refine replace
The 3R concept in animal research: Reduce Refine, and Replace should be known to anyone involved in preclinical research. Every research strategy should at least take the 3Rs into account. We’ve given a lot of examples to replace or reduce animal testing that you can use for your drug development. However, not all examples are available for each development program. To learn what may apply to you, contact TRACER. Furthermore, the 3Rs not only benefit animal welfare but also contribute to more efficient, cost-effective drug development. Shortening timelines, lowering costs, and improving chances of success. Receive an estimation for your possibilities, including a timeline and budget; ask our preclinical experts.
Frequently asked questions
Below we answer some specific questions on 3R in animal research. These answers go into a bit more detail on this topic. If your question is not listed, but you want to know how you can apply 3R animal research, feel free to contact us.
What is 3R research? And where does it come from?
The basis for 3R research has been initiated by W. M. S. Russell and R. L. Burch in the book “The Principles of Humane Experimental Technique”, published in 1959. Since then, 3R research has been widely adopted in regulatory and scientific guidelines. To give a few examples:
- FDA, ICH, and the 3Rs.
- EMA Regulatory acceptance of 3R (replacement, reduction, refinement) testing approaches – Scientific guideline.
- In addition to regulatory agencies, there are country-specific institutions promoting the 3Rs. To list a few examples: NC3Rs UK, 3Rs Centre Utrecht (3RCU), FC3R France and NORECOPA Norway.
What are the 3Rs of animal research? And what has been the impact?
What are the 3 Rs in research? The 3Rs stand for Replacement, Reduction, and Refinement of animals in research. These objectives can be achieved by the techniques and methodologies described in this article. Regulatory guidelines related to laboratory animal research include the 3Rs. Governments show commitment to harmonizing regulations and encouraging innovation in this area. The FDA Modernization Act 2.0 is a good example of this. However, we still have a long way to go.
Looking at the statistics in the EU, we can conclude that the yearly number of laboratory animals has remained almost constant over the past decade, between 8-10 million. In the US mice, rats, birds, and fish are excluded from the Animal Welfare Act (AWA).
What is 5 R in animal research?
In addition to the 3Rs animal testing, two more Rs have been proposed and have already been adopted in some countries: Reuse, and Rehabilitate. Reuse encourages determining if an individual animal may be used continuously, repeatedly, or reused. For example, the same animal used for a preclinical study that resulted in mild symptoms can be used for a new study. The 5th R stands for Rehabilitate, where the possibility of rehabilitation should be considered. For example, instead of euthanizing laboratory dogs, they can be adopted by a loving family. Both the 4th and 5th R have to be assessed on a case-by-case basis and depend on the outcome of the previous investigation.