The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) provides ICH M3 R2 guidelines for non-clinical safety studies. In the ICH M3 guideline 5 approaches to exploratory clinical trials for microdosing and non-microdosing studies are described. The ICH M3 Guidelines cover study requirements related to dose limitations, preclinical pharmacology and toxicity. For drug developers, the most useful is the table with an overview of exploratory clinical trial approaches.
Download exploratory trial approaches table
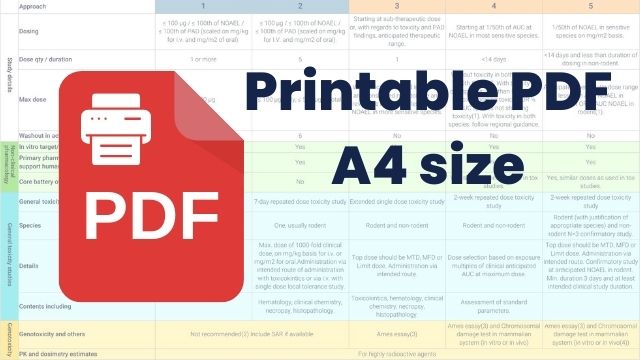
ICH M3 Exploratory trial approaches
The exploratory clinical trials in the ICH guidelines can be divided into three main categories.
- Microdose studies and non-microdose studies.
- Single and multiple-dose clinical trials.
- Intended route of administration or via intravenous injection (even if the intended route differs).
Not covered by the ICH approaches but worth mentioning, is the distinction between exploratory clinical trials with imaging and without imaging.
Contact TRACER to discuss imaging for your clinical trial.
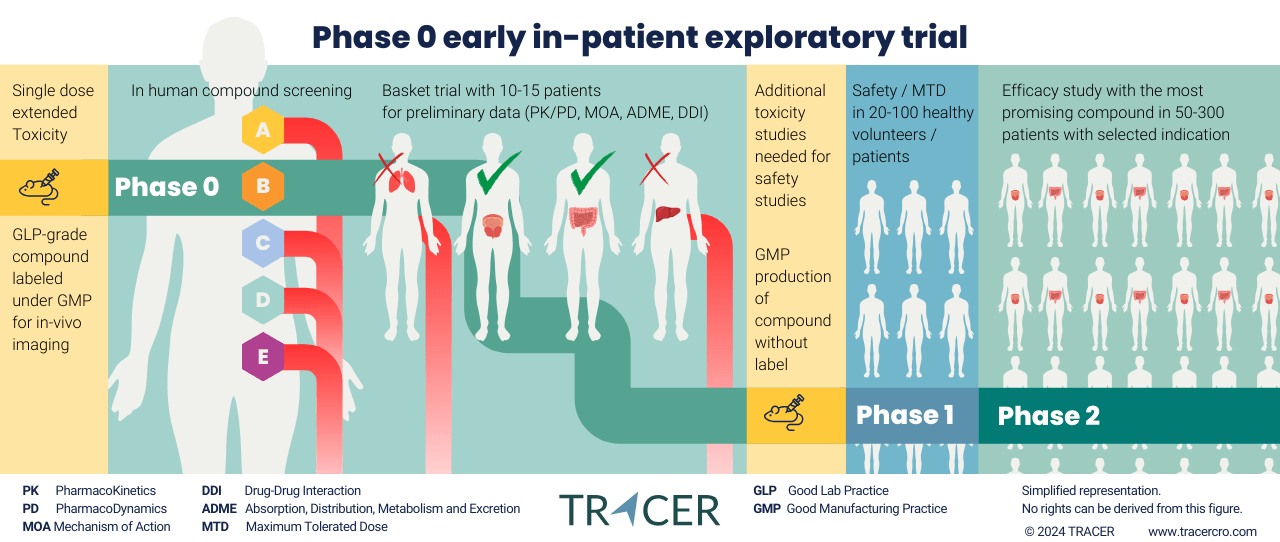
What are exploratory clinical trials?
Exploratory clinical trials replace part of the preclinical stage with early in-patient trials. This new approach has been initiated by regulatory authorities, such as the FDA and EMA, and guidelines have been harmonized by the ICH. Preclinical requirements to move into humans have become more lenient when only a small dose is used.
Exploratory studies allow drug developers to obtain data in their target population 1-2 years earlier than the conventional trajectory. This should result in a better selection of lead compounds and their targets. By eliminating less-promising compounds earlier, resources in clinical trials are made available for compounds with a higher rate of success.
Exploratory trial approaches as defined by ICH M3 guidance
The ICH defines 5 approaches as examples, but other study-specific clinical trial designs are possible.
(scroll right →)
|
|
|
Microdose |
Non-microdose |
|||
|
|
Approach |
1 |
2 |
3 |
4 |
5 |
|---|---|---|---|---|---|---|
| Study details |
Dosing |
≤ 100 μg / ≤ 100th of NOAEL / ≤ 100th of PAD (scaled on mg/kg for i.v. and mg/m2 of oral). |
≤ 100 μg / ≤ 100th of NOAEL / ≤ 100th of PAD (scaled on mg/kg for I.V. and mg/m2 of oral). |
Starting at sub-therapeutic dose or, with regards to toxicity and PAD findings, anticipated therapeutic range. |
Starting at 1/50th of AUC at NOAEL in most sensitive species. |
1/50th of NOAEL in sensitive species on mg/m2 basis.
|
|
Dose qty / duration |
1 or more |
5 |
1 |
<14 days |
<14 days and less than duration of dosing in non-rodent. |
|
|
Max dose |
≤ 100 μg |
≤ 100 μg daily, ≤ 500 μg in total. |
With toxicity observed in animals and considered monitorable and reversible in humans: less than ½ NOAEL in more sensitive species. |
Without toxicity in both species: 1/10th the AUC.
With toxicity in one species less than NOAEL of species showing toxicity, OR ½ AUC species not showing toxicity(1).
With toxicity in both species, follow regional guidance. |
Anticipated therapeutic dose range less than AUC NOAEL in non-rodent OR ½ AUC NOAEL in rodent(1). |
|
|
Washout in actual or predicted half-lifes |
No |
6 |
No |
No |
No |
|
| Non-clinical pharmacology |
In vitro target/receptor profiling |
Yes |
Yes |
Yes |
Yes |
Yes |
|
Primary pharmacology in a PD relevant model to support human dose selection |
Yes |
Yes |
Yes |
Yes |
Yes |
|
|
Core battery of safety pharmacology |
No |
No |
Yes |
Yes, similar doses as used in tox studies. |
Yes, similar doses as used in tox studies. |
|
| General toxicity |
General toxicity studies |
14-day extended single dose toxicity study |
7-day repeated dose toxicity study |
Extended single dose toxicity study |
2-week repeated dose toxicity study |
2-week repeated dose toxicity study |
|
Species |
One, usually rodent |
One, usually rodent |
Rodent and non-rodent. |
Rodent and non-rodent |
Rodent (with justification of appropriate species) and non-rodent N=3 confirmatory study. |
|
|
Details |
Via intended route of administration. |
Max. dose of 1000-fold clinical dose, on mg/kg basis for i.v. or mg/m2 for oral. Administration via intended route of administration with toxicokinetics or via i.v. with single dose local tolerance study. |
Top dose should be MTD, MFD or Limit dose. Administration via intended route. |
Dose selection based on exposure multiples of clinical anticipated AUC at maximum dose. |
Top dose should be MTD, MFD or Limit dose. Administration via intended route. Confirmatory study at anticipated NOAEL in rodent. Min. duration 3 days and at least intended clinical study duration. |
|
|
Contents including |
|
Hematology, clinical chemistry, necropsy, histopathology. |
Toxicokintics, hematology, clinical chemistry, necropsy, histopathology. |
Assessment of standard parameters. |
|
|
| Genotoxicity |
Genotoxicity and others |
Not recommended(2) Include SAR if available |
Not recommended(2) Include SAR if available |
Ames essay(3) |
Ames essay(3) and Chromosomal damage test in mammalian system (in vitro or in vivo) |
Ames essay(3) and Chromosomal damage test in mammalian system (in vitro or in vivo(4)) |
|
PK and dosimetry estimates |
For highly radioactive agents |
For highly radioactive agents |
For highly radioactive agents |
For highly radioactive agents |
For highly radioactive agents |
|
- Whichever is lowest.
- Any studies or SAR assessments conducted should be included in the trial application.
- Or alternative assay if Ames is inappropriate, such as for an antibacterial product.
- In vivo assessment can be part of the rodent toxicity study.
Abbreviations
| AUC | Area Under the Curve |
| I.V. | Intravenous |
| PAD | Pharmacologically Active Dose |
| NOAEL | No Observed Adverse Effect Level |
| MTD | Maximum Tolerated Dose |
| MFD | Maximum Feasible Dose |
| SAR | Structure-Activity Relationship |
Download this table

General considerations regarding ICH M3 exploratory clinical trials
All Phase 0 approaches are not intended to evaluate MTD but can be used to calculate MTD. The therapeutic range should be lower than MTD. Depending on preclinical toxicity outcomes, regional guidance on initial human dosing should be considered. In certain cases, some studies may be necessary, modified, postponed, or omitted. Review ICH S6 for guidance on biologically derived products, such as vaccines, growth factors, immune modulators, and monoclonal antibodies. Other cases include innovative therapeutic modalities and new medicines for life-threatening or serious diseases without current therapy.
Maximum tolerated dose is not always needed in animal studies
In animal toxicology studies, it is not always needed to extend the research to MTD. Other dose limitations can be used when considered appropriate to predict clinical safety. Limited doses in toxicology studies can include MFD, saturation, or exposure to multiples. The same applies to studies with pre-existing limits on dose. One of the objectives of the ICH M3 R2 guideline is to reduce the use of animals in preclinical trials. Further preclinical research can be discontinued for compounds that fail in the in-human exploratory phase. By only using successful compounds in higher doses, preclinical research can continue after developers have already shown promise in humans in low doses.
About TRACER
TRACER is an imaging CRO specialized in early phase clinical trials with patients. We allow drug developers to take advantage of the exploratory clinical trial framework. This includes Phase 0 first in-patient studies, proof of concept studies, Phase 1, and Phase 2a pilot studies. For drug developers, we offer knowledge sessions completely free of charge. You can book this session here. In about 30-60 minutes you’ll learn all about the possibilities available to speed up drug development: from regulatory requirements to compound labeling for imaging.
Frequently asked questions
Below we answer questions regarding the ICH M3 document. These questions relate to the application of the ICH M3(R2) guidance document. An ICH M3(R2) questions and answers document is available on the ICH website to answer in-depth questions on the contents of the guidance.
For questions on clinical trials, clinical translation, or preclinical toxicity studies, simply contact us at TRACER.
What is M3 in ICH?
The M in ICH M3 refers to Multidisciplinary Guidelines, where 3 is the number of the guideline: M3 Non-Clinical Safety Studies. This document handles the non-clinical safety studies for the conduct of human clinical trials and marketing authorization for pharmaceuticals.
What is ICH M3 R2?
When a revision is made to an ICH guideline, the letter R with a subsequent number is added to the document title. In the case of ICH M3 R2, it is the second revision. The ICH M3 R2 guideline came into effect in December 2009. At the time of publishing this article, M3 R2 is the current effective version.
Is ICH M3 FDA approved?
Yes, you will find the ICH guidelines also on the FDA website. The ICH provides guidelines that are used in drug development worldwide. Safety, quality, and efficacy for new medicinal products are harmonized between regions, including Europe, the USA, and Japan.
Does EMA follow ICH guidelines?
Yes, the scientific guidelines published by the European Medicines Agency (EMA) are harmonised by the ICH. This makes the regulatory guidelines the same for all EU member states. This applies to both the European Union and the European Economic Area. The Committee for Medicinal Products for Human Use (CHMP) of the EMA provides technical and scientific support to the ICH.
ICH guideline M3 R2:
https://www.ema.europa.eu/en/ich-m3-r2-non-clinical-safety-studies-conduct-human-clinical-trials-pharmaceuticals-scientific-guideline
Although this article has been composed with great care and attention, we cannot guarantee its accuracy. If you have any suggestions or additions to this article, please email info@tracercro.com.
No rights can be derived from this publication. This blog does not make claim or promote ownership to any intellectual property, study information, clinical images, or copyrighted terms wherein.