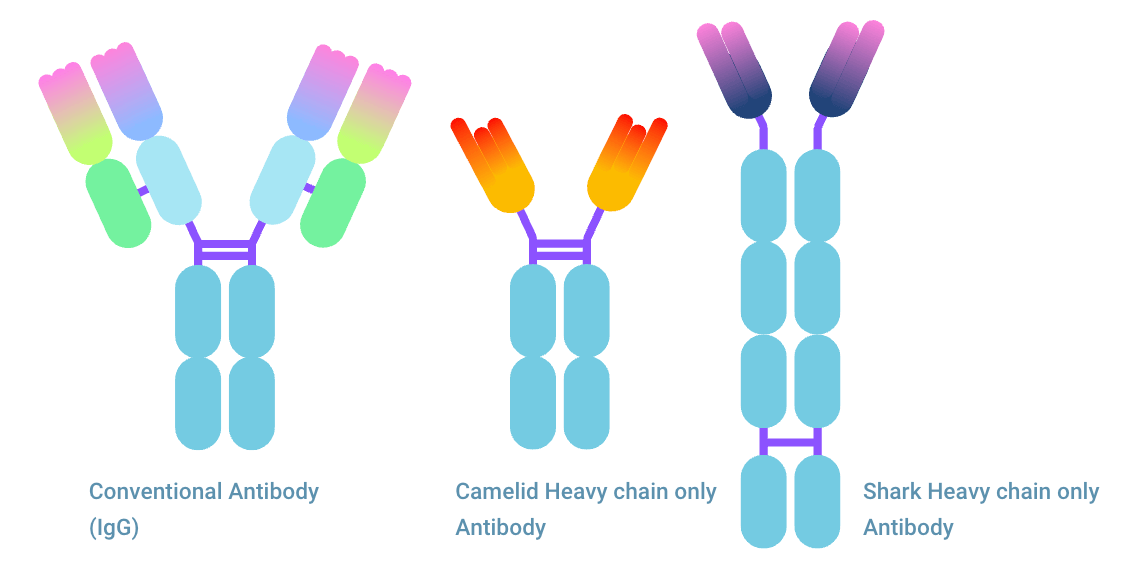
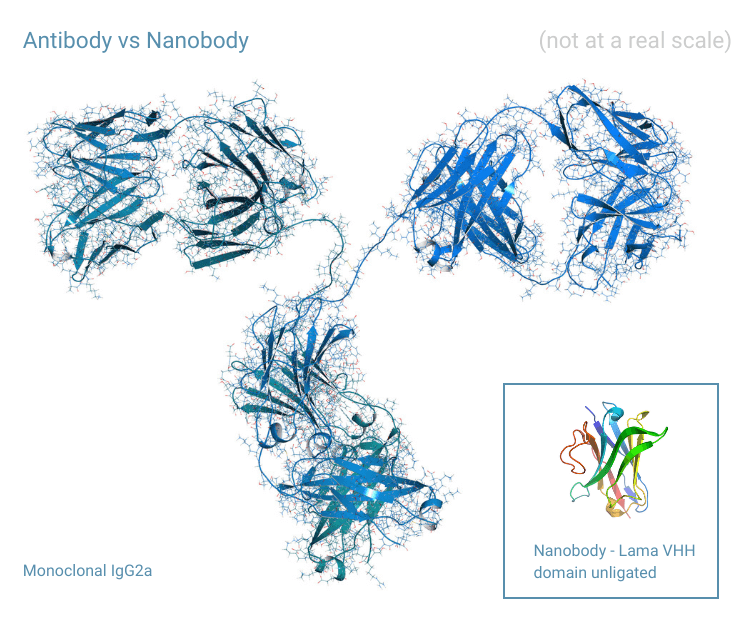
The comparison nanobody® vs antibody has become more relevant as nanobodies® are making their entrance into clinical use. Nanobodies® (Nbs) are variable domain of heavy chain only antibodies (HCAbs). HCAb consists of two heavy chains (HCs) and lacks a light chain (LC). Therefore, the antigen binding part is only one fragment. This binding fragment named VHH nanobody®, originates from camelids. When the nanobody® is named VNAR, they are shark nanobodies®. This monomeric protein can, similar to that of conventional antibodies, be produced for therapeutic and diagnostic usage. But how do nanobodies® compare to antibodies (Abs) and antibody fragments? In this article, you’ll learn everything about it.
Fact list of nanobodies® compared to antibodies
The one thing you need to consider when comparing antibody vs nanobody® is that nanobodies® are the smallest inartificial antigen binding fragments. They have various advantages when compared to antibodies and there is overlap in usage. This fact list gives you basic information needed to understand the value of nanobodies® compared to antibodies.
Article sections
This article is divided into four sections.
1. The first section will cover the comparison of characteristics.
2. The second section will discuss the benefits of antibody vs nanobody® and vice versa.
3. The third section will show examples of nanobodies® and considerations for clinical trials.
4. The fourth section contains an FAQ.
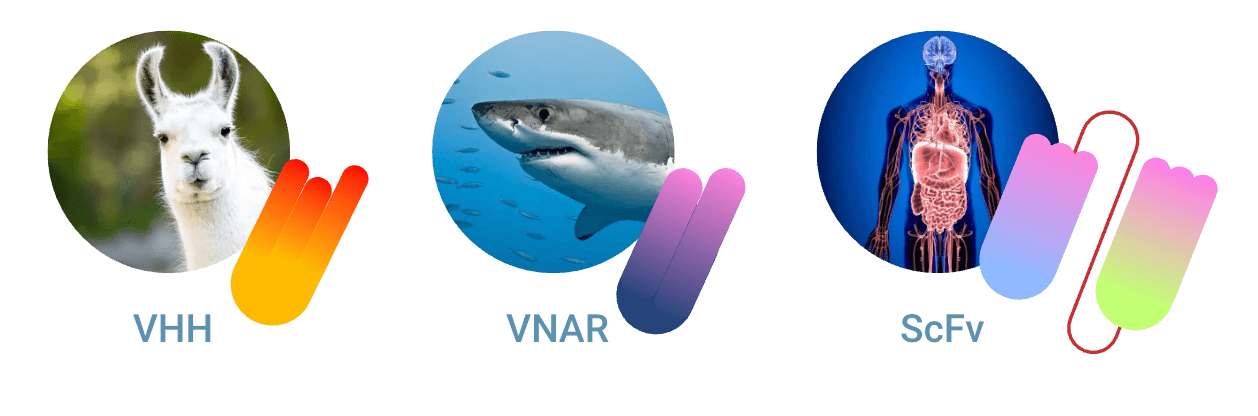
There are two known types of nanobodies®
1. The VHH nanobody® abbreviation comes from Variable domain of Heavy chain of Heavy-chain only antibody.
2. VNAR is derived from Variable New Antigen Receptors and is the variable domain of IgNAR.
| Type | Nb | Ab |
| Size nm diameter / length: | 2.5 / 4 | 14.2 / 11-14 |
| weight kDa | 12 – 15 | 150 (for IgG) |
| half-life | .5 – 2 h | days to weeks |
| CNS access | Both for Nb and Ab solely by engineering | |
| Intercellular access | Easier | More difficult |
| Trigger immune response | No | Yes |
| Quantity of therapeutics on market | <<5 | 800> |
| Pathogen clustering | Not without engineering | Yes |
| Glycosylation sites | Rare/Engineered | Yes, quantity depending on Ab isotype |
| Complementary Determining Regions (CDR) | 3 (VHH) 2 (VNAR) | 6 |
Nanobody® vs antibody: Basic facts
- The VHH antibody structure only has a heavy chain, while a conventional antibody has a heavy chain and a light chain.
- Nanobody® size nm: 2.5 diameter, 4 in length, in comparison, IgG antibody size can be around 14.2 nm in diameter and approximately 11-14 nm in length.
- Nanobody® molecular weight: 12 – 15 kDa, antibodies are approximately 150 kDa (in case of IgG type).
- Nanobody® half-life is around 0.5 – 2 h, for Ab’s this is several days to weeks. The short half-life for Nb’s is one of the main disadvantages when it comes to nanobodies® used for therapeutics.
- Nb’s provide fast and deep penetration into tissue and fast distribution.
- A nanobody® normally forms disulfide bonds, but generally less than an antibody. The exact amount for an antibody depends on the number of cysteines in the sequence of the antibody type. For example, an IgG1 antibody contains 16 disulfide bonds.
- The ability to reach the Central Nervous System (CNS) for both antibodies and nanobodies® is still under investigation.
- Without alteration, antibodies cannot cross the Blood Brain Barrier (BBB) for nanobodies® this is still being evaluated. Some studies report BBB passage of Nb’s (with GFP imaging). .
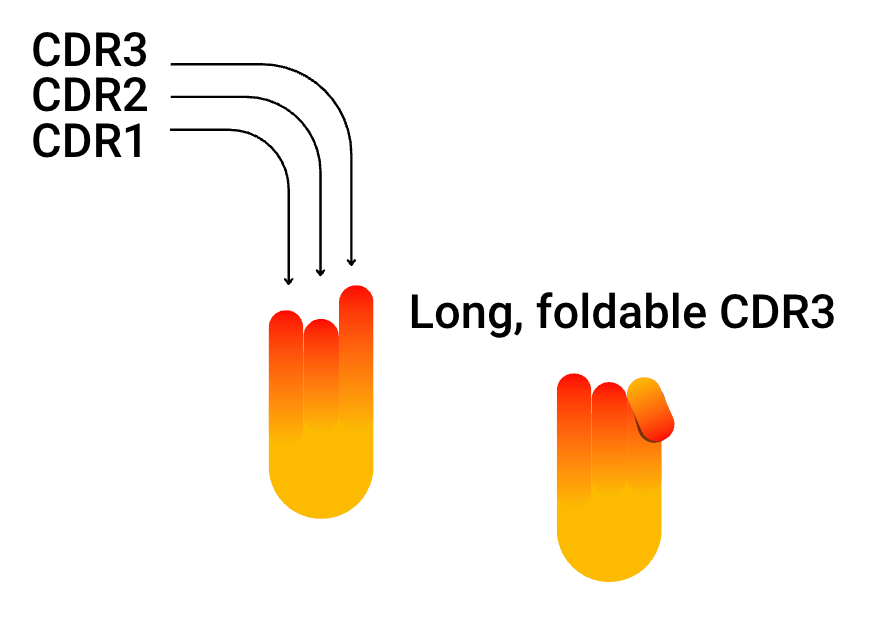
- Nanobodies® have an extended Complementary Determining Region 3 (CDR3) in comparison to the CDRs of common human antibodies that have a relatively flat antigen binding surface.
For the record: VHH vs VH vs VNAR
Nanobody® vs antibody can be seen as an incomplete and imperfect comparison. Namely, the counterpart of the nanobody® in a conventional antibody is the single-chain Fragment variable (scFv) (VH – VL domain). We do not compare VHH vs VH, since isolated human VH domains (without VL domains) are not (or are very difficult) to use as biologics.
Furthermore, a nanobody® refers officially to VHH. VHH is the antigen binding fragment of camelid antibody HCAb. VNAR is also considered a nanobody® structure. This is the antigen binding fragment from shark heavy chain only Ab, named IgNAR. We are aware of this subdivision, and therefore we compare in this paragraph VHH vs scFv vs VNAR.
Nanobody® vs scFv
The most important aspects of nanobody® vs scFv are listed below. Nanobody® is separated into VHH and VNAR.
| VHH | VNAR | scFv | |
| Complementary-determining regions | 3 | 2 (lacks CD2) | 2×3 (3 CDRs on VL & VH) |
| Molecular weight | 15 | 12 | 30 |
| Disulfide bond | Yes | Yes | Yes / peptide linker |
| Origin | Camelid | Shark / Cartilaginous fish | Mammals (and all species with heavy-light chain Ig) |
| Yield in recombinant expression (comparatively) | Higher | Unknown | Lower |
Differences nanobody® vs scFv
In general, there are many advantages of nanobody® over scFv due to chemical and physical properties. We compare nanobody® vs scFv below:
| Type | Nanobody® | scFv |
| Epitope binding | Grooves and clefts | Flat linear |
| Act as scaffold | Yes | Yes |
| Solubility (comparatively) | Higher | Lower |
| Stability (comparatively) | Higher | Lower |
| Production cost (comparatively) | Lower | Higher |
| Bond | Disulfide bond (commonly) | Flexible linker |
| Half-life in blood | 0.5 – 2 hours | 5 – 6 hours |
| Penetration in tissue (comparatively) | Higher | Lower |
| Diversity in paratope (comparatively) | Higher | Lower |
| Affinity (comparatively) | Equal | Equal |
| Possibility to enhance affinity (comparatively) | Higher | Lower |
| Intracellular targeting | Higher | Lower |
The key difference in antigen binding: the long CDR3 “finger”
The major difference in VHH and VNAR vs scFv is the longer CDR3 loop. Antigen cavities that are not reachable for any other antibody variable region can be reached by the “finger-like” CDR3 of VHH and VNAR. It can even fold, a mechanism that compensates for the lack of a light chain. The direction of the folding CDR3 corresponds to the direction where the VH would find support from its VL counterpart.
Imaging CRO for (pre)clinical imaging of antibodies and nanobodies®
TRACER is an imaging CRO specialized in Phase 0 clinical trials. We conduct clinical studies for any type of compound, including antibodies, fragments, and nanobodies®. As an imaging CRO we closely observe the behavior of a drug in-vivo by using various molecular imaging techniques.
By labeling a compound with a radioactive label, PET or SPECT imaging generates data on pharmacokinetics (PK) and biodistribution (BD) and allows tracking of a compound in-vivo over time. Another in-vivo imaging tool is optical imaging, labeling with a fluorescent dye or a fluorophore. Antibodies, and nanobodies® in particular, offer exciting new possibilities in both therapeutic and diagnostic applications.
About this blog and about TRACER
As an imaging CRO, we are pioneering clinical trials and working together with sponsors who have the same mindset. We are scientifically driven and are conducting research in our area of expertise. Writing blogs on these subjects is a part of that. We hope our blog will educate you as a drug developer on the topic of drug development and offer information for your (pre) clinical trials. To discuss the possibilities for your compound in person, our scientists are happy to meet. Contact us so we can schedule a meeting.
Contact TRACER
List of benefits nanobodies® vs antibodies
- Nanobodies® can recognize and reach targets that traditional antibodies cannot reach.
- Nb’s offer strong binding to antigens.
- Nanobodies® offer superior stability and solubility compared to conventional antibodies.
- They are chemically stable over a larger pH range than antibodies.
- They offer a wider variety of antigen binding sites and have the capability to bind on more cryptic epitopes than conventional Ab’s.
- Nb’s are easy to engineer.
- No mismatch error when creating bispecific antibodies (bsABs) due to the absence of VH and VL chain construction.
- The possibility to chain multiple nanobodies® together (multimerization).
- Nanobodies® have a better thermostability compared to antibodies (up to 70 degrees centigrade/Celsius) and for VNAR even slightly higher.
- The benefit above also makes nanobodies® easy to produce; purification can be done by heating up the bacteria lysates.
- Nanobodies® can be produced by microbial cells, bacteriophages can be of use for variant development.
- Nanobodies® can be recombined with a linker (linking at the DNA level) that allows for fluorescent probes and radiotracers.
- Low immunogenicity risk profile.
- Able to pass the kidney’s renal filter for rapid blood clearance.
On these factors, nanobodies® and antibodies (or Ab fragments) are similar
- Both can be converted into different formats, like Fc-Fusion Proteins (FcFP) and heterodimers or can be used in various conjugated formats.
- Both are promising in many therapeutic areas, including oncology, neurodegenerative diseases, infectious diseases, inflammation, envenoming, and toxicity.
Benefits antibody vs nanobody®
- The VL fragment of conventional antibodies can provide stability and binding specificity.
- There is at this moment much more available data from antibody studies in the literature.
- There are many existing and proven antibody therapeutic and diagnostic applications.
- Antibodies can trigger immune effector functions due to their natural occurrence in the human body.
- Their large size and structure can be beneficial when targeting certain epitopes.
- Certain clinical situations can benefit from a longer half-life.
- A broad range of antibody fragments and platforms are available.
- Many antibody labeling kits are available and validated for antibodies.
- Already widely established in terms of regulatory approval.
- There are many antibody isotopes, subtypes, and allotypes.
Nanobody® therapeutics examples
You can find a few examples of nanobody® drugs below. Click on the name to read more.
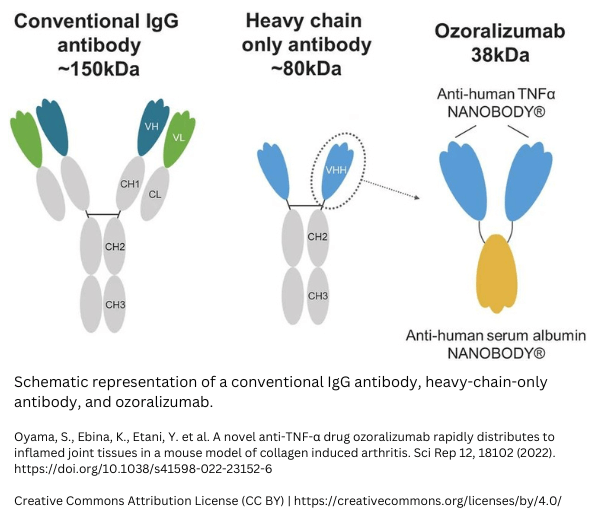
Ozoralizumab
Ozoralizumab is an anti-TNFα-nanobody®. It is a humanized trivalent antibody that consists of two nanobodies® targeting TNF-alpha and one anti-human serum albumin nanobody®. The binding to TNF-alpha aims to neutralize, while the binding to serum albumin aims to extend the half-life. A study comparing Ozoralizumab to Adalimumab (IgG antibody) showed that traditional anti-tumor necrosis factor alpha antibodies lead to anti-drug antibodies (ADAs), while Ozoralizumab did not. Read the whole study here: https://www.frontiersin.org/articles/10.3389/fimmu.2022.853008/full.
Caplacizumab
In 2018, vWF Caplacizumab received market authorization from EMA and in 2019 from the FDA. Good to know, the first nanobody® FDA approved was Caplacizumab. To make the comparison to conventional antibody fragments, it was only the eleventh antibody fragment approved by the FDA. The nanobody® drug binds to the A1 domain of von Willebrand Factor (vWF). The drug is used for aTTP (acquired Thrombotic Thrombocytopenic Purpura), a rare blood disorder related to the immune system.
Example study: HER2 nanobody® – Trastuzumab
The difference between antibodies and nanobodies® clearly shows in studies involving a HER2 nanobody®. A HER2 nanobody® will meet tough competition in the form of Trastuzumab. This mAb against human epidermal growth factor receptor 2 (HER2) was already approved by the FDA back in 1998. Trastuzumab triggers an immune response and is blocking the HER2 pathway. Still, investigations are ongoing on HER2 nanobodies®. Benefits are expected due to their small size and are therefore thought to offer deeper tumor penetration. For imaging of HER2 breast cancer, the fast clearance of radiolabeled HER2 nanobodies® could beat the slow clearance of HER2 antibodies. However, there are also disadvantages for radiolabeled nanobodies®. One of the disadvantages is the accumulation in the kidneys.
Studies anti-HER2 Antibodies vs nanobodies®
Several studies are evaluating anti-HER2 antibody drug conjugates (ADCs). Many of those are conjugated to Trastuzumab. The same applies to the diagnostic use of HER2 antibodies. Research is ongoing on radiolabeled trastuzumab (zirconium-89, chelator DFO (deferoxamine)). Some takeaways from completed research are:
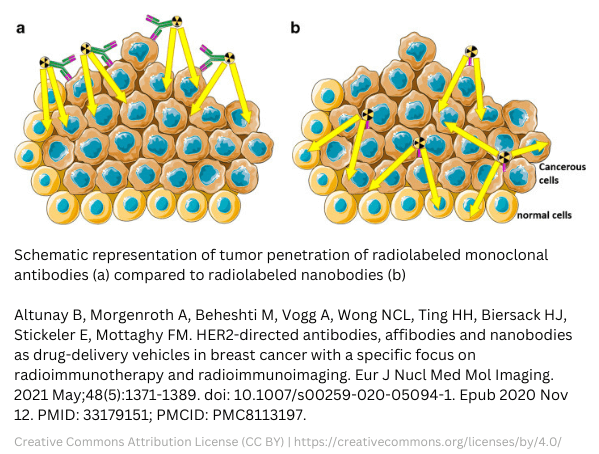
- HER2 nanobodies® can overcome issues with slow blood clearance, which is a current issue with HER2 antibodies for PET or SPECT imaging.
- Nanobodies® can offer a high background-to-tumor ratio. Studies regarding nanobody®-Lutetium-177 with different chelators already showed promising results.
- On the other hand, the dose delivered to the tumor by 177Lu-DTPA-trastuzumab was 6-fold higher than with nanobody®-Lutetium-177. However, adverse effects occur due to longer circulation and slower excretion in lung, liver, spleen, bone, and blood. For more information, view studies regarding this subject here: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8113197/
What to consider for antibodies and nanobodies® in clinical trials?
There is great interest in antibodies and nanobodies® for diagnostics and therapeutics. TRACER specializes in molecular imaging clinical trials to bring those compounds to patients faster. From study setup and dealing with regulations up to full study closure. Following on from the studies discussed earlier, there are specific considerations for nanobodies® and antibodies. In particular, the collection of PK/BD data and on-target and off-target accumulation.
Both for antibodies and nanobodies® there are a few things to consider for clinical trials. We’ve listed them below.
Imaging clinical trials for antibodies and nanobodies®
- Both antibodies and nanobodies® work on the basis of binding to a receptor, an imaging trial can show if binding occurs to the target tissue.
- When combined with a CPP, nanobodies® can enter cells and can bind to intracellular targets. (https://www.mdpi.com/1424-8247/14/7/602).
- Especially when targeting tumors, using a basket trial design can give valuable insights regarding several indications.
- Phase 0 is a fast translational clinical trial that usually comes before Phase 1, it allows skipping large animal models and the clinical trial trajectory can be started with GLP material.
- Most clinical trials fail (around 90%) and one major contributing factor is the differences between drug behavior in-animal and in-human. Since nanobodies® are always non-human and antibody discovery is done mostly in animals, there is a risk of species mismatch.
- Imaging clinical trials are not limited to Phase 0, they can also be conducted in Phase 1 or 2. As the examples in this article show, this can relate to analyzing clearance, half-life, uptake, and off-target accumulation.
Factors related to the use of radionuclides for SPECT or PET clinical trials
- Antibody labeling should not lead to impairment of binding or effector mechanisms.
- Due to the small size of nanobodies®, finding the right site for attaching the nanobody® label for imaging can be more challenging compared to antibodies.
- When radionuclides are used in SPECT or PET clinical trials, the half-life of the imaging agent should match the half-life of the antibody or nanobody®.
- Choosing the right radionuclide and chelator for labeling antibodies, antibody fragments and nanobodies® is important. The method, number of linked chelators per antibody or changes in the radiolabeled molecule can lead to changes in biodistribution and binding affinity.
- Quality control method should be implemented to ensure the quality of the radiolabeled antibody or nanobody®.
Discuss with TRACER your preclinical to clinical translation and your ideal study design for your clinical trial.
Clinical trials for Antibody Drug Conjugate (ADC)
We can first only label the antibody (or nanobody®) to ensure binding to the target before continuing with the complete clinical development of an ADC. When you use an antibody or nanobody® that has no proven binding to your target antigen in humans, consider this method to ensure binding first. One very important factor to be aware of; when an antibody, antibody fragment, or nanobody® is conjugated with a drug, it is important to choose an imaging agent that binds to the drug. In case the drug gets separated from the nanobody® or antibody, you want to know where it accumulates. It is also possible to image both drug and antibody and to follow the ADC over time.
Antibodies vs nanobodies® in imaging
The use of antibodies for imaging is influenced by their long half-life. Depending on the type of antibody, clearance can take somewhere between a few days to several weeks. Due to the long circulation of antibodies, you will reach a target-to-background ratio, sufficient for imaging after several days or a week. The long circulation time of the radiolabeled antibody also results in a higher radiation burden to the patient. The large size of antibodies gives limitations on tissue that can be reached. Nanobodies® decrease the radiation burden for the patient due to fast clearance.
A glance at the future: Development of artificial antibodies and nanobodies®
The current changes in antibody nomenclature for INN underline the future of monoclonal Antibody (mAb) development. The major change was the replacement of the -mab suffix with four more specific subfixes indicating the mAb type. One of the suffixes is -bart, which indicates artificial antibody. For nanobodies® artificial development is already in progress with sybodies, which stands for synthetic nanobodies®. The method for artificial nanobodies® and antibodies is fully in-vitro, without the use of animals.
Conclusion
Antibodies have been a huge discovery and with over 880 monoclonal antibodies in clinical use today, they are a major success. Nanobodies® were discovered relatively recently, and clinical application is still under investigation. On the other hand, nanobodies® show great promise in terms of therapeutic and diagnostic use. Their advantages perfectly complement the limitations of antibodies. When looking at nanobody® vs antibody, the number one benefit is the small size of nanobodies®. The second is the long CDR3. The third benefit is the greater ease of production. Only the future can tell what useful nanobody® applications will reach the market.
Would you like to discuss the preclinical or clinical development of your antibody or nanobody®? Contact us, we are happy to schedule a call.
Contact us
Resources for your study
When conducting research in nanobodies®, these recourses might be helpful. If you have a suggestion, please let us know.
INDI and iCAN nanobody® database
iCAN nanobody® is a database of the Institute Collection and Analysis of Nanobodies®. It was the first database on nanobodies® and antigen binding. It is available here: http://ican.ils.seu.edu.cn.
The INDI antibody database stands for Integrated Database of Nanobodies® for Immunoinformatics. It has a smart method of combining data from multiple sources based on the variable sequence of the nanobodies®. You can find the database here: http://research.naturalantibody.com/nanobodies®
More resources are available! Contact us for more research material regarding your study.
FAQ on Nanobody® vs Antibody
For a fast understanding, or to check the knowledge you’ve gained from this article, read the FAQ on this topic.
What are nanobodies®?
Nanobodies® are the variable components of the heavy chain only antibody. There are two known species in which this immunoglobulin type occurs, these are camelids and sharks (cartilaginous fish). The antigen binding fragment of camelid HCAb is named VHH and has a molecular weight of approximately 15 kDa. A similar fragment in sharks is named VNAR and is coming from immunoglobulin IGNAR. The molecular weight of this variable fragment is slightly lighter, around 12 kDa. Both are referred to as nanobody®, although that word itself is a trademark from Ablynx.
What are the advantages of nanobody® over antibody?
The advantages of nanobody® over antibody mostly relate to their smaller size. The size allows them to target antigens and bind to receptors that are not available for full size conventional antibodies. Other advantages include a wide range of pH and temperature stability and ease of production. An advantage of antibodies over nanobodies®, is their natural ability to trigger an immune response. Nanobodies® can be engineered with conventional antibodies for a similar mechanism of action, but size advantages are thus partially eliminated.
What is the difference between monoclonal antibody and nanobody®?
The difference between monoclonal antibody vs nanobody® is the same as for a natural antibody in comparison with a nanobody®. Monoclonal simply means that a specific antibody is cloned. Nanobodies® can also be cloned for therapeutic, diagnostic, or research practices. While nanobodies® are only the antigen binding part, a conventional monoclonal antibody is full length. However, nanobodies® can be engineered in a monoclonal antibody. On the other hand, components of a full-length antibody can be engineered in different formats. The antigen binding part of a conventional antibody is used as Fab fragment, scFv fragment, or a different engineered structure. A major benefit of monoclonal antibodies vs nanobodies® is the huge amount of data and research already available on the former.
What is the difference between a heavy chain antibody and a nanobody®?
The answer to this question comprises several facets. First of all, there is a difference between a heavy chain antibody and heavy chain only antibody. Conventional antibodies comprise heavy chains and light chains. A heavy chain only antibody lacks light chains. Therefore, a nanobody®, the antigen binding fragment of the heavy chain only antibody, can bind as a single component. A construct of heavy and light chain binding fragments is therefore not needed to bind to an antigen. There can be differences in binding capability and affinity between nanobodies® and conventional antibodies. So, in short, the difference between a heavy chain antibody and nanobody®, is that the latter is only the antigen binding part. The former is the full-length construct.
How big is a nanobody® compared to an antibody?
The molecular weight of a VHH nanobody® originating from camelids is 15 kDa. The IgNAR nanobody®, originated in sharks has a molecular weight of 12 kDa. In comparison, a conventional antibody, IgG isotype weighs 150 kDa. An IgE antibody weighs 190 kDa and IgD has a weight of 180 kDa. IgA weighs in monomer form 160 kDa. In dimeric formation, the IgA consists of two IgA conjugated by J-chain. IgM pentamer consists of 5 full length antibodies connected with a J-chain and weight 900 kDa. A hexamer IgM weighs even more since the J-chain is replaced by another full length antibody.
Please note, that all weights and measurements are approximate. Various studies can show slightly different numbers.
NANOBODY® compound is a registered trademark of Ablynx N.V.
Abbreviations
| Ab | Antibody |
| ADA | Anti-Drug Antibody |
| ADCs | Antibody Drug Conjugates |
| aTTP | acquired Thrombotic Thrombocytopenic Purpura |
| BBB | Blood Brain Barrier |
| BD | BioDistribution |
| BsAb | Bispecific Antibody |
| BsNb | Bispecific Nanobody® |
| CDR | Complementarity-Determining Regions |
| CRO | Contract Research Organization |
| CNS | Central Nervous system |
| DFO | Deferoxamine |
| FAB | Fragment Antigen-Binding |
| FcFP | Fc-Fusion Proteins |
| GFP | Green Fluorescent Protein |
| GLP | Good Lab Practice |
| GPCR | G Protein-Coupled Receptor |
| HA | Hemagglutinin |
| HC | Heavy Chain |
| HCAb | Heavy Chain only antibody |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| IgG | Immunoglobulin G |
| IgH | Immunoglobulin Heavy chain |
| INN | International Nonproprietary Names |
| kDa | kilodalton |
| LC | Light Chain |
| mAb | monoclonal Antibody |
| MOA | Mechanism Of Action |
| Nb | Nanobody® |
| Nm | nanometer |
| PK | PharmacoKinetics |
| POI | Protein Of Interest |
| scFv | single-chain Fragment variable |
| TNF | Tumor necrosis factor |
| VNAR | Variable New Antigen Receptor |
| vWF | von Willebrand Factor |