In recent years, more types of antibodies, also referred to as immunoglobulin types, have been discovered. Now we know there are more varieties than the 5 commonly known human antibody isotypes (IgG, IgA, IgM, IgE, and IgD). (Fragments of) new antibody types can be used in (monoclonal) antibody development for therapeutic or diagnostic use. A nanobody®, the binding fragment of the recently discovered heavy chain-only antibody, is a great example. Due to antibody drug development, there is an increased interest in the different types of antibodies. When you’re a drug developer or working in this field and want to learn more about (pre)clinical trials with antibodies, contact our experts.
Contact TRACER
Scope of this article: antibody types
This blog provides a list of antibodies beyond the most known 5 types of antibodies. Types of antibodies are defined by the natural structure of their heavy chains. Antibodies are in their standard structure composed of two identical polypeptide chains named heavy chains and two identical, shorter polypeptide chains named light chains. There are several types of light chains, but these do not affect the name of the antibody type. The antibody types discussed in this article are based on naturally occurring structures and not on engineered antibodies or antibody fragments.
Structures of antibody fragments for research and drug development are outside the scope of this article but have recently been reviewed. Read more about antibody fragments.
Heavy chain-only antibodies, as the name already indicates, lack light chains and are currently the only known exception to the standard structure.
Why are isotypes, subtypes, allotypes, and idiotypes important for drug developers?
- Antibody isotypes have different functions
- Newly discovered antibody isotypes can be beneficial in research and drug development
- Antibody subclasses have slightly different structures and functions
- Antibody allotypes can trigger an immunogenetic response
- Antibody idiotypes can lead to anti-idiotypic (Anti-ID) antibodies
Antibody vs immunoglobulin
The words antibody and immunoglobulin are often used interchangeably. Strictly speaking, an immunoglobulin is attached to the B-cell and an antibody is freely circulating. The B-cell becomes a plasma cell through activation via, among others, the immunoglobulin. It then produces antibodies with the same binding structure as the original immunoglobulin. However, these terms are used interchangeably.
Antibody isotypes, subtypes, allotypes and idiotypes
To indicate the structure of antibodies, they are divided into tiered types. The main structures from top to bottom are isotypes, subtypes, isoforms, allotypes, and idiotypes. Each isotype has several subtypes, and each subtype can have different isoforms. For example, isotype IgG, has four subtypes IgG1, IgG2, IgG3, and IgG4, and the subtype IgG2 has three isoforms IgG2A, IgG2A/B, and IgG2B. In addition to this, there are minor differences in structure, named allotypes and idiotypes.
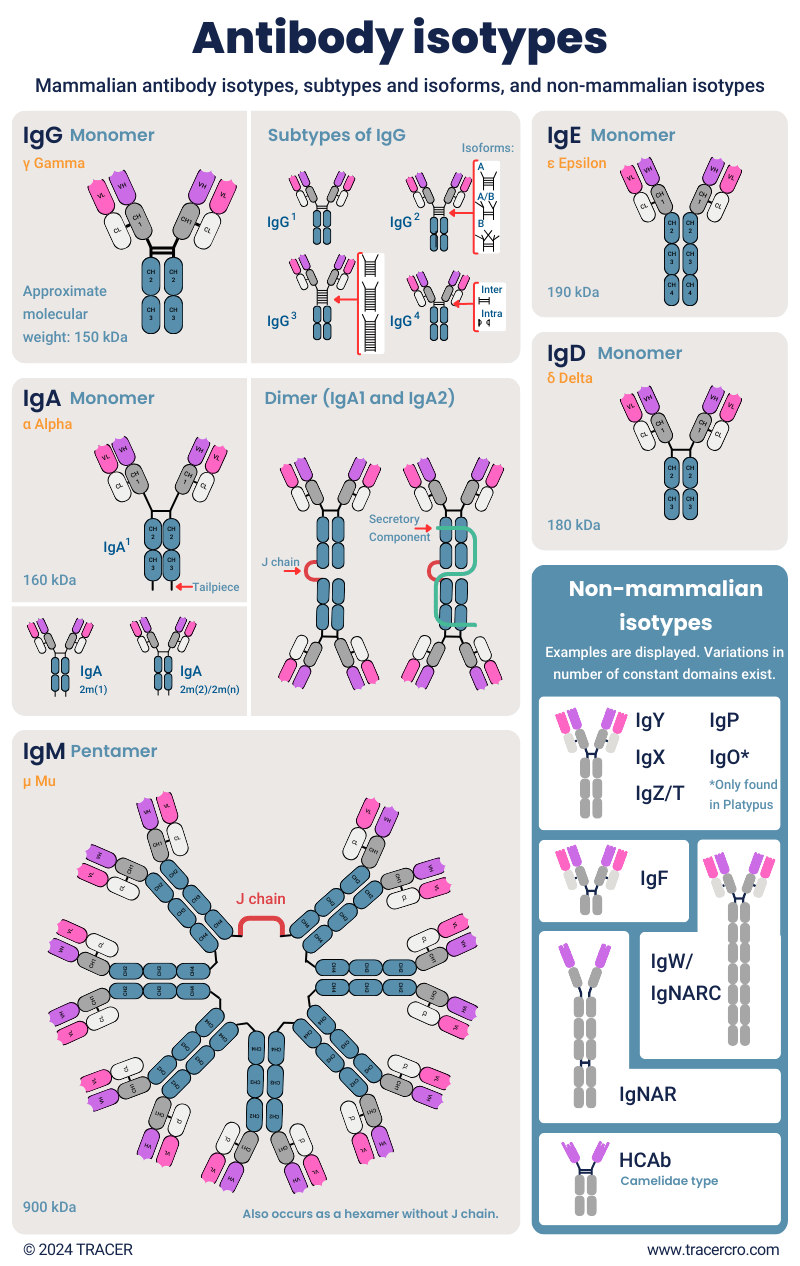
Download Antibody Types poster
After submitting, you will receive the pdf directly in your inbox.

Antibody isotype
The top-level category is antibody isotype and is defined by the structure of the constant domain, the heavy chain. Isotype naming is based on the heavy chain since it determines the structure and immune effector function, such as the complement system. Antibody naming format is Ig for Immunoglobulin, followed by an indicator. For example, mammalian antibodies have isotypes IgG, IgA, IgM, IgE, and IgD. Additional isotypes exist in other species.
Antibody light chain isotypes
Worth mentioning is that there are two common isotypes of antibody light chains: kappa (κ), and lambda (λ). A third and fourth light chain of sigma (σ) and σ-cart, occurring only in fish, were discovered more recently. Since no major difference in function has been discovered yet, the isotype of light chains doesn’t affect the antibody isotypes definition.
Antibody subtypes or subclasses
Subtypes or different classes of antibodies are defined by minor differences in the heavy chain or the hinge between the chains. For example, the heavy chain of IgW can range from 2 to 11 constant domains. Effector functions can be different for subclasses. Commonly, subtypes are indicated with a number. IgG has four subclasses, IgG1, IgG2, IgG3, and IgG4. IGA exists as IgA1 and IgA2. IgD has subclass IgD1 and IgD2.
Isoforms
Related to subtypes are isoforms, indicated with e.g., A/A, B/B, or A/B to indicate structural differences between subclasses. For example, these differ in the amount or design of disulfide bridges. Isoforms are different versions of a protein that are derived from the same gene but have unique amino acid sequences. It is important to check for these variations in antibody production since they can change the functional properties.
Antibody allotypes
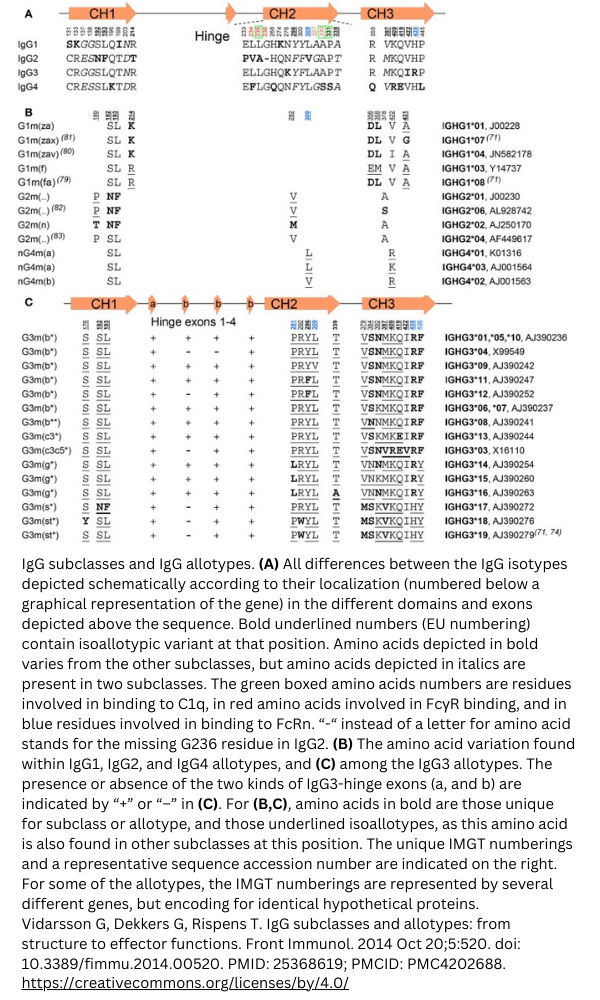
Antibody allotypes, allotypic determinants, are variations in the constant domains of the heavy chains and light chains and can differ per individual within species. These allotypes are indicated with an “m” for marker. For example, IgA2 has three recognized allotypes, IgA2m1, IgA2m2, and IgA2mn. A recent study [1] found 20 allotypes for IgG and 3 for IgA. Three allotypes were found on the IgG kappa light chain. Specific combinations of allotypes are referred to as haplotypes.
Example: IgG allotypes
View the IgG subclasses and allotypes from this study [2] below.
Antibody idiotypes
Antibody idiotypes are defined as the combination of idiotopes, the antigenic determinants, in the Variable domain (V). The idiotopes are the individual parts of the Complementary Determining Region (CDR) that allow the antibody to bind to the epitope/antigen. The part of the antibody that can bind to the epitope, is also named paratope. The binding can be referred to as epitope-paratope interaction. In drug development, this interaction can be structured and used for machine learning of antibody-antigen combinations. In anti-idiotypic antibodies, existing as antibody drugs, antibody-antibody binding is possible by idiotope binding. In that case, the paratope can become the epitope.
About TRACER
TRACER is an imaging CRO, meaning we specialize in utilizing imaging modalities in clinical trials. We couple compounds, such as antibodies with an imaging agent to make a compound suitable for imaging in (pre)clinical trials. The same can apply to Antibody Drug Conjugates (ADCs). Our CRO services offer drug developers valuable information on the behavior of the antibody by visualizing biodistribution and pharmacokinetics. Our Phase 0 proof-of-concept studies allow drug developers to conduct a clinical trial in-patient before Phase 1, with minimal preclinical work. Contact us to discuss possibilities for your antibody in preclinical, translational, and clinical drug development.
First in-human clinical trials for Antibody-drug conjugates
For ADCs, we can replace the drug with a radionuclide for nuclear imaging in first-in-human clinical trials. Imaging allows you to visualize the whole-body biodistribution of your ADC. Moreover, this makes it possible to predict side effects based on off-target accumulation without the risk of potential toxic effects caused by your ADC. First targeting and the mechanism of action of your ADC are established, before attaching the toxic payload or drug. There are many possibilities to get data on on- and off-target binding and the biochemical reaction. Together with our experts, you can discuss the best approach for your ADC.
How many types of antibodies are there?
Before we answer the question of how many types of antibodies there are, you need to understand that new types are continuously discovered. Therefore, any antibody type list will eventually be incomplete. As mentioned before, the number of 5 types of antibodies is already outdated. Let’s look at a few examples of discovered antibodies and their clinical use.
Heavy chain-only antibody (HCAbs)
In 1989 Professor Raymond Hamers, of the Vrije Universiteit Brussel (VUB), discovered the heavy chain-only antibody (HCAbs) in camelids. His discovery was published in 1993 [3]. The antibody binding fragment of HCAbs is named nanobody®, VHH, or single domain antibody (sdAb). The small size of nanobodies® comes from the lack of a light chain. Nanobodies® are already in drug development, Caplacizumab is even already on the market.
Nanobodies®, binding part of HCAb
Single domain antibodies don’t use a light chain counterpart. Drug developers can use this to reach deeper tissue and bind epitopes inaccessible by antibodies with a light chain. The deep penetration and fast clearance of nanobodies®, make them interesting for radiopharmaceuticals in diagnostics and therapeutics. The molecular weight of nanobodies® is only 15 kDa. In comparison, this is half the weight of scFv (Single Chain Fragment Variable) fragments, the smallest binding fragment of conventional antibodies.
IgNAR
In 1995, Andrew Greenberg discovered a type of heavy chain-only antibody in sharks[4] similar to HCAb camelid antibodies. The antigen binding fragments of IgNAR, named VNAR, are also categorized as nanobodies®. They differ in the number of CDR regions: camelid nanobodies® have three CDRs and shark nanobodies® have two. VNAR offers a higher stability than camelid nanobodies®. VNAR drugs are currently in development but did not yet obtain market approval at the time of writing.
IgY antibodies
In 1893 Klemperer made the discovery of IgY in egg yolk [5], but the name IgY, Igυ for upsilon or ypsilon, was given to this antibody in 1969 by Leslie and Clem [6]. The “Y” stands for “Yolk”. Although discovered in birds, IgY is also found in reptiles, amphibia, and lungfish. IgY is considered the equivalent of mammalian IgG. In structure they are different, IgY contains four constant domains, whereas IgG only contains three. IgY benefits in medicine are ease of production, no animal harm, no activation of the complement system, no binding to Fc receptors or common binding proteins, stability, and in engineering similar to IgG. Several IgY-based therapeutics are currently under development.
IgYΔFc
An isoform of IgY found in ducks is IgYΔFc. This truncated IgY lacks the last two constant domains that allow opsonization and complement fixation for the full IgY. An advantage of this could be that it can neutralize without activation.
List of antibodies
In this immunoglobulin types list you’ll find antibody isotypes with their molecular weight and origin. Information on immunoglobulin types not yet mentioned in this article is given below the antibodies isotype list.
|
Antibody Isotype |
Latin name |
CH domains |
Molecular Weight (kDa) |
Y of discovery |
Origin / Species |
|
IgA |
Igα |
3 |
Approx. 160 |
1959 |
Mammals, Birds, Reptiles, Amphibians |
|
IgD |
Igδ |
3 |
Approx. 180 |
1965 |
Mammals, Birds, Reptiles, Fish, Amphibians |
|
IgE |
Igε |
4 |
Approx. 190 |
1967 |
Mammals |
|
IgG |
Igγ |
3 |
Approx. 150 |
1960s |
Mammals |
|
IgA |
Igα |
Approx. 160 (monomeric)/385 (sIgA) |
1959 |
Mammals, Amphibians, Birds |
|
|
IgM |
Igμ |
4 |
Approx. 900 (pentamer) |
1960s |
Mammals, Birds, Reptiles, Fish, Amphibians |
|
IgY |
Igυ |
2/4 |
Approx. 170-180 |
1969 |
Birds, Reptiles, Amphibians |
|
IgX |
– |
4 |
Estimated 105 |
1985 |
Cartilaginous Fish, Amphibians |
|
IgT |
Igτ |
2-4 |
Approx. 180 |
2005 |
Teleost Fish |
|
IgZ |
Igζ |
2-4 |
– |
2005 |
Fish |
|
IgW/IgNARC |
Igω |
– |
2-11 |
1984/1990/1996 |
Cartilaginous Fish |
|
HCAb (Heavy Chain-Only Antibodies) |
– |
2 |
Approx. 75 |
1989 |
Camelids |
|
IgNAR (shark Immunoglobulins of HCAb type) |
– |
5 |
– |
1995 |
Cartilaginous Fish |
|
IgF |
Igφ |
2 |
– |
2006 |
Amphibians |
|
IgP |
– |
4 |
– |
2008 |
Pleurodeles (Amphibian) |
|
IgO |
– |
4 |
– |
2009 |
Platypus (Ornithorhynchus anatinus) (Mammal) |
- A dash (-) indicates we could not find suitable data.
- Please note that new IgG types may be discovered posterior to the publication date of this table.
- Number of constant heavy chain (CH) domains or occurrence in species is an indication.
- Immunoglobulin types found in different species can be orthologous, sharing a common ancestral origin and often similar functions.
Antibody isotypes discovery
Below we continue the list of antibody isotypes with information on the discovery.
IgT and IgZ
In 2005 John D. Hansen published an article [7] on the discovery of the IgT antibody in rainbow trout. The “T” stands for teleost fish and can also be written as tau or “𝜏”. In the same year, the immunoglobulin zeta (IgZ) was discovered by Nadia Danilova, Jeroen Bussmann, Kerstin Jekosch, and Lisa A Steiner [8]. Where the “Z” refers to “Zebrafish”. IgT and IgZ are very similar. For consistency purposes, the scientific community recommends the use of IgT. You may also find IgT/Z, to be used to indicate this immunoglobulin type. IgT and IgZ are not limited to teleost and zebrafish and can also be found in other species.
Subclasses of IgT and IgZ
IgT/IgZ subclasses have also been found. IgT1, IgT2, and IgT3 are examples of that. Abbreviations can also use the Greek alphabet, Igτ1, Igτ2 and Igτ3. Two subtypes of IgT/Z in common carb have been identified as IgZ1 and IgZ2, where the first has four constant domains and the second only has two.
IgX
The IgX antibody was published in the article “A third immunoglobulin class in amphibians” by E. Hsu, M. F. Flajnik, L. Du Pasquier in 1985 [9]. The name IgX comes from Xenopus laevis, South African frog.
IgF antibodies
IgF antibodies were identified by Yaofeng Zhao in 2006 [10]. Each arm of the IgF protein only consists of two constant domains. Development of therapeutics or diagnostics using Yaofeng Zhao’s discovered IgF antibody could not be found at the time of writing.
IgP
The IgP antibody type was discovered in 2008 by Bérénice Schaerlinger [11]. The “P” stands for the species in which the antibody has been found; the Iberian Ribbed Newt (Pleurodeles Waltl). When you conduct research in IgP, keep in mind that this abbreviation can also be used for Immunoglobulin Protein. At the time of writing, we could not find Immunoglobulin P in drug development.
IgW antibodies (IgNARC antibodies)
IgW has been discovered multiple times by different people. IgW is sometimes referred to as IgNARC and was upon discovery referred to as IgR or IgX. IgW was discovered in 1984 by Kunihiko et al. [12] [13], in 1990 by Harding et al. [14], by Berstein et al. in 1996 [15], and IgNARC was discovered by Greenberg et al. in 1996 [16]. After the initial discovery, scientists have found different forms of IgW. A study from 2014 [17] suggests two additional immunoglobulin isotypes originated from IgW and indicated as IgN and IgQ.
IgNARC vs IgNAR
IgNARC should not be confused with IgNAR, the first stands for Ig New Antigen Receptor from Cartilaginous fish, while the second only means Ig New Antigen Receptor. IgW (IgNARC) is very different from IgNAR, where IgNARC follows the common heavy-light chain structure, IgNAR lacks the light chain.
IgO
IgO was discovered in 2009 by Yaofeng Zhao [18]. This immunoglobulin isotype was presented as the sixth mammalian antibody type. The “O” stands for “Ornithorhynchus anatinus”, Platypus. The IgO structure contains four constant heavy domains.
IgH, IgL, IgC, IgV, IgI, IgK, and IgR
Abbreviations for antibodies can be confusing. Abbreviations you commonly encounter, and their meaning are listed below. While it may look like they indicate different types of immunoglobulins, they refer to domains.
– IgH: Immunoglobulin Heavy chain
– IgL: Immunoglobulin Light chain OR Immunoglobulin Lambda (λ) locus
– IgC: Immunoglobulin constant domain (IgC1, IgC2, IgC3, IgC4)
– IgV: Immunoglobulin Variable domain
– IgI: Immunoglobulin Intermediate domain
– IgK: Immunoglobulin Kappa (κ) locus
– IgR: Immunoglobulin Receptors (meaning B-cell membrane-bound immunoglobulins serving as receptors)
– IGLV: meaning Immunoglobulin Lambda Variable
Download Antibody Types poster
After submitting, you will receive the pdf directly in your inbox.

Frequently asked questions
Below we answer some specific questions on immunoglobulin types. For other questions, don’t hesitate to contact us.
What are immunoglobulin types?
Immunoglobulin types are categorizations of antibodies based on structure and function.
What the different types of immunoglobulin are, depends on the scope of the question. Common criteria of categorization are:
- Immunoglobulin structure and function: receptor or circulating antibody, neutralizer, agglutination, precipitation, complement system activation.
- Heavy chain type, subtype, allotypes, and idiotypes.
- Location in the body: for example, blood, mucosal areas, secretions.
- Membrane-bound, monomeric, dimerization, or polymerization capabilities.
- Origin: mammals, birds, cartilaginous fish, teleost fish, birds, amphibians, and reptiles. Some antibodies have only been found in specific animals.
- Types of antigen: for example: exogenous or endogenous antigens, bacteria, viruses, cancer cells etc.
What are 5 types of antibodies?
The immunoglobulin isotypes that occur naturally in mammals are the commonly known 5 isotypes. These 5 exclude the recently found IgO in Platypus. The five types of antibodies are IgG, IgA, IgM, IgE, and IgD. Remember this by putting the letters together, forming the word GAMED. The word GAMED does not state the sequence in which the antibodies take effect when a pathogen is encountered. In addition to these 5 types of antibodies, there are more (non-mammalian) antibodies.
What are the two main types of antibodies?
What the main types are, depends on the criteria. Common answers to this question are: The two main types of antibodies are membrane-bound and circulating antibodies. But also, the two main types are monomeric or polymeric antibodies. If you look at what the most abundant type of immunoglobulin is in humans, the two main types are IgG and IgA.
Some interesting facts (to share with your colleagues during the next lunch)
To conclude this article, we will end with some interesting facts.
- There are at least 16 antibody isotypes, but more may be discovered in the future.
- It was long believed that mammals only had 5 antibody types, but a sixth one, IgO, was discovered in Platypus in 2009.
- Immunoglobulin types found in different species can be orthologous, sharing a common ancestral origin and often similar functions.
- When writing about IgT or IgZ, the scientific community prefers IgT for consistency purposes since this is a broadly used term.
Antibody antigen bindings are often described as a key in a lock, but in reality, there is not just one match, there are different degrees of affinity binding. Depending on the shape complementary, it can be a weak fit or a perfect fit, with the last one resulting in high affinity.
Find out how the affinity of your antibody is in patients
TRACER can answer a very simple question for you:
Does your antibody reach its target, is it able to bind to it, and does it function as intended?
You’ve probably already tested this in lab experiments with good results. However, the human body provides your antibody with new challenges. And of course, there is the aspect of safety in clinical trials. At TRACER we work according to a simple approach: Phase 0. This clinical trial is conducted with a microdose of your compound in a small group of patients. By doing so, we can provide early-efficacy indicating data in about 14 months. Simultaneously, we can prepare your Phase 1 clinical trial to assess safety before moving to Phase 2.
Citations
Yaofeng Zhao, Huiting Cui, Camilla M. Whittington, Zhiguo Wei, Xiaofeng Zhang, Ziding Zhang, Li Yu, Liming Ren, Xiaoxiang Hu, Yaping Zhang, Lars Hellman, Katherine Belov, Ning Li, Lennart Hammarström; Ornithorhynchus anatinus (Platypus) Links the Evolution of Immunoglobulin Genes in Eutherian Mammals and Nonmammalian Tetrapods12. J Immunol 1 September 2009; 183 (5): 3285–3293. https://doi.org/10.4049/jimmunol.0900469
Although this article has been composed with great care and attention, we cannot guarantee its accuracy. If you have any suggestions or additions to this article, please email info@tracercro.com.
No rights can be derived from this publication. This blog does not make claim or promote ownership to any intellectual property, study information, clinical images, or copyrighted terms wherein.